By Natalya Ortolano
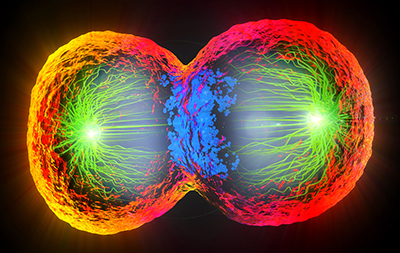
When a bouncy ball deforms under the weight of your body, its rubber membrane stretches and contracts. Likewise, the membrane of a cell doubling itself prior to division must accommodate changes in intracellular pressure — or explode.
Protein complexes known as myosin motors play a central role in the process through their effect on the cortex, a specialized layer of proteins on the cell’s inner-facing membrane. Myosin motors and long filaments composed of the protein actin accumulate at the center of the cell and constrict the membrane. Intracellular pressure builds as the membrane pinches off into two distinct cells. To relieve that intracellular pressure, a membrane deformation known as a bleb forms at the polar ends of the cell.
In a study recently published in Cell Reports, a team of researchers led by Nilay Taneja, a graduate student in the lab of Dylan Burnette, assistant professor of cell and developmental biology, determined that during cell division, two distinct myosin motors — myosin-IIA and myosin-IIB — mediate cortex tension and bleb formation at the cell cortex.
Previous studies implicated MIIA, and not MIIB, in bleb formation during division. However, using human cells in a dish, Taneja’s team determined that both MIIA and MIIB affect bleb formation at the polar ends of the cell. MIIA-depleted cells exhibited decreased tension, while MIIB-depleted cells had increased tension. When the scientists manipulated intracellular pressure, cells with reduced levels of MIIA blebbed less, while cells with lower MIIB levels produced a greater number of larger blebs. Depletion of both myosin motors yielded a single cell with two nuclei instead of the expected two daughter cells.
The authors conclude that both MIIA and MIIB contribute to blebbing and normal cell division and regulate the process in different ways. Other model organisms such as worms and flies contain only one type of MII, which implies that MIIA and MIIB diverged later in evolution, yet upstream regulation of MII is conserved from flies to mammals. The authors comment that future studies may reveal how MII regulates cortex tension in these cells. Further understanding of MII regulation and function in these organisms may provide insight into why mammalian cell division requires both MIIA and MIIB.
This research was funded by the National Institute of General Medical Sciences and the American Heart Association.