Triple-negative breast cancer, an aggressive type of breast cancer, accounts for 10 percent of all breast cancer cases in the United States annually. CD8+ T cells that normally kill cancer cells often become exhausted and stop. To overcome that, some cancers, such as TNBC, can be treated with immune checkpoint inhibitors, which inhibit checkpoint proteins that cause T-cell exhaustion and allow them to continue to attack tumor cells. Certain ICIs have been approved for use in TNBC in combination with standard-of-care chemotherapy, but the patient response is variable and often not durable, and there is evidence that many common chemotherapeutic drugs have, in fact, immunosuppressive effects.

To tackle this problem, Ann Richmond, professor of pharmacology, is optimizing chemotherapy or targeted therapy approaches for use in combination with ICI.
In their latest research paper, published in the journal, Cancers, Richmond lab members Kennady Bullock and Patricia Ward, in collaboration with researchers from the High-Throughput Screening Facility Thomas Hasaka, Emily Days, and Joshua Bauer, screened a unique library of anticancer compounds that are either currently in clinical trials or that have been approved by the U.S. Food and Drug Administration to see if they could enhance the CD8+ T-cell-mediated killing of tumor cells.
We sat down with Richmond to talk about the recent paper.

What issue/problem does your research address?
Therapies currently used to treat cancers often also inhibit the ability of immune cells, such as CD8+ T-cells, to kill tumor cells. The purpose of our study was to identify currently available drugs used for cancer therapy that could enhance the ability of CD8+ T-cells to kill tumor cells.
To accomplish this, we developed a screening assay to test whether any of 448 anticancer compounds (part of a library provided by the High-Throughput Screening Facility) could enhance the ability of CD8+ T-cells to kill cancer cells. We cultured CD8+ T-cells that recognize a specific antigen expressed by breast cancer cells and monitored their ability to kill tumor cells in the presence of each of the compounds. Since these drugs also directly kill tumor cells, we treated a second set of cells with the drugs but without the CD8+ T-cells and used that to infer how much tumor cell death was due to the drug-enhanced activity of the CD8+ T-cells versus the drug alone.
What was unique about your approach to the research? Was anything about the work unique to Vanderbilt University?
The first author, Kennady Bullock, developed a unique assay to detect drug-enhanced CD8+ T-cell killing of tumor cells. In addition, the methodology used to monitor T-cell-mediated tumor cell killing was developed by the HTS facility.
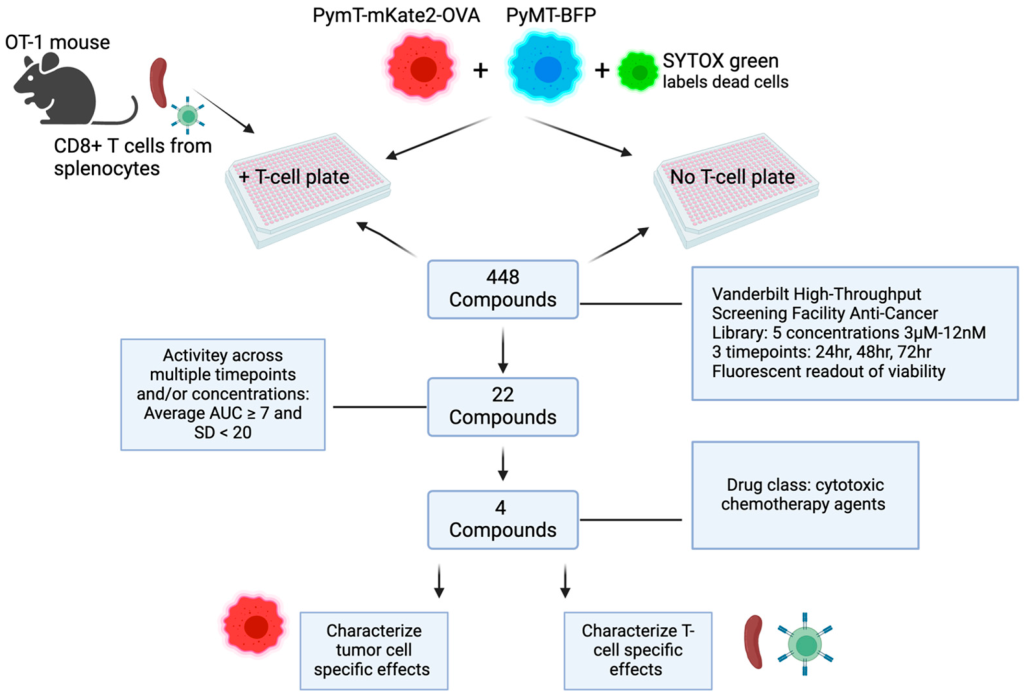
What were your top findings?
We screened more than 400 drugs and identified 22 that potentiated the ability of CD8+ T-cells to kill breast cancer tumor cells. We focused additional attention on the four drugs with the greatest T-cell-enhancing activity: paclitaxel, bleomycin sulfate, ispinesib, and etoposide.
These lead compounds affected the immunogenicity (the ability to elicit an immune reaction) of the tumor cells, which was reflected in their increased expression of three markers involved with antigen presentation, MHCI, MHCII, and PD-L1, and their release of two markers of immunogenic cell death, ATP and HMGB1.
Who or what made the difference in your research? What small things contributed to your work?
This research resulted from Kennady’s creativity when she was a graduate student in the lab. That said, the HTS facility made it possible to achieve the results we got.
What do you hope will be achieved with this research on the short term?
Although paclitaxel is already used for treatment of certain breast cancers, often in combination with the checkpoint inhibitors anti-PD1 or anti-PDL1, our data indicate that bleomycin sulfate, ispinesib, and etoposide may also increase the patient response to these immune checkpoint blockade therapies. Moreover, our results show that these T-cell-enhancing drugs could be used at doses low enough to potentiate the CD8+ T-cell killing of tumor cells without some of the toxic side effects that normally accompany the higher doses needed for drug-induced death of the tumor cells. Using these drugs to potentiate the immune-mediated killing of cancer cells could make for a less unpleasant and toxic chemotherapy experience for patients.
What are your highest translational or clinical aspirations that might result from this research?
We hope that this assay can help identify other drugs that, at low doses, can potentiate T-cells to better kill tumor cells and work in concert with immune checkpoint inhibitors to avoid toxic side effects for cancer patients.
Where is this research taking you next?
Kennady is looking to follow up with this work and continue studying how the immune system can be harnessed to treat cancer during her current postdoctoral work in the laboratory of Dr. Patrick Hwu at the Moffitt Cancer Center.
Go deeper
The paper “A High-Throughput Immune-Oncology Screen Identifies Immunostimulatory Properties of Cytotoxic Chemotherapy Agents in TNBC” was published in Cancers in December 2024.
Funding
This research used funds from the National Cancer Institute, the National Institutes of Health, and the U.S. Department of Veterans Affairs.
Shared resources
This research made use of the High Throughput Screening Facility.