Research
For cellular life to exist, genetic material must be copied and passed on to newly divided cells. In humans, this process is phenomenally accurate and occurs with an error rate of around one in a billion. In the Dewar lab, we are working to understand the mechanisms that underlie the completion of DNA synthesis, which poses unique problems that are not present during earlier stages of replication. Our research is important to better-understand molecular causes of aging and cancer, develop better cancer treatments, and evaluate the risk of genotoxins (both environmental and endogenous).
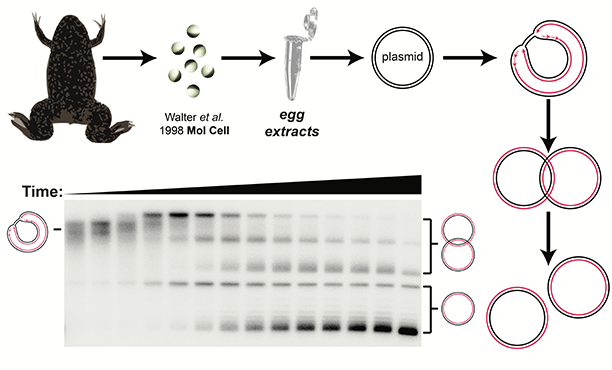
Approach
The Dewar lab’s primary approach is to use cell extracts derived from eggs of the African clawed frog, Xenopus laevis (‘Xenopus egg extracts’). This system allows us to study DNA replication and DNA repair in a test tube using the full set of vertebrate DNA replication proteins, which are shared with humans. We can therefore use Xenopus egg extracts, to monitor DNA replication and DNA repair with extraordinary sensitivity and resolution in vitro, even without knowing all of the proteins involved. Additionally, we can employ proteomics to determine which proteins are involved in the processes we study. Overall, Xenopus egg extracts allow us to study complex molecular mechanisms of DNA replication and repair in a high level of detail and perform unbiased analyses of the proteins involved. We also complement our in vitro approaches with in vivo experiments using human cells.