Meet GRKs and Arrestins
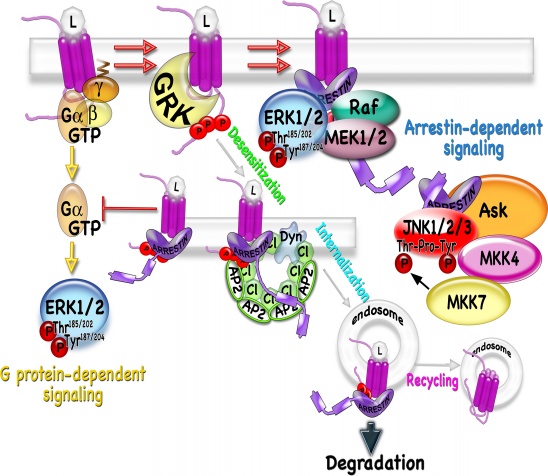
Arrestins and GRKs mediate desensitization, trafficking, and signaling of G protein-coupled receptors (GPCR). The ligand (L) binding to GPCRs activates the G protein-mediated signaling. GRKs phosphorylate active GPCRs inducing high affinity arrestin binding. Arrestin binding precludes further receptor-G protein interaction and terminates G protein-mediated signaling. By scaffolding the elements of the signaling cascade, arrestin initiates alternative signaling events such as activation of MAP kinases (MAP3K-MAP2K-MAPK). Arrestin interacts with major components of coated pits, clathrin (Cl) and AP2, via binding sites located on the C-tail (shown as rectangles on the arrestin C-tail) and recruits the receptor to coated pits, thus promoting dynamin (Dyn)-dependent receptor internalization. In endosomes, ligands dissociate, internalized receptors are dephosphorylated, and either recycled back to the plasma membrane or send to lysosomes to be degraded.
G protein-coupled receptors (GPCRs) are the largest known family of signaling molecules, encoded by ~1,000 genes out of ~20,000 in the human genome. The main signaling pathway initiated by GPCRs is remarkably uniform. The active receptor catalyzes GDP/GTP exchange on a cognate heterotrimeric G protein. GTP binding induces release of the G protein from the receptor and its dissociation, whereupon the GTP-liganded a-subunit activates or inhibits the appropriate effectors. As soon as the signal goes through, the signaling machinery needs to be shut down. This is accomplished by a mechanism of signal termination shared by the great majority of GPCRs: receptor phosphorylation by a G protein-coupled receptor kinase (GRK) followed by the binding of an uncoupling protein termed arrestin. Arrestins serve as adaptors, mediating internalization of desensitized receptors by virtue of their interaction with the major components of the coated pits, clathrin and AP-2. Arrestin bound to active phosphorylated GPCRs redirects the signaling from G protein-mediated to G protein-independent pathways (such as MAP kinase pathways) by acting as a scaffold for the assembly of multiprotein signaling complexes. Thus, arrestins and GRKs are critical regulators of signaling events in cells, integrating G protein-mediated and G protein-independent pathways.
The seven mammalian GRK isoforms are classified into three subfamilies based on sequence homology and biochemical properties. GRK1 and 7 (rhodopsin kinase and cone opsin kinase, respectively) comprise the visual GRK subfamily and are largely confined to the retina. Of non-visual GRK isoforms, GRK2 and 3 belong to one subfamily, whereas GRK4, 5, and 6 belong to another. The level of GRK4 in the brain is very low, and it is mostly found in the cerebellum. Four GRK isoforms, GRK2, 3, 5, and 6, are ubiquitously expressed throughout the brain and appear to interact with multiple GPCRs. Out of four mammalian arrestin isoforms, two, visual and cone arrestins, are expressed almost exclusively in the retina. The remaining two non-visual isoforms, arrestin2 (a.k.a. b-arrestin1) and arrestin3 (a.k.a. b-arrestin2) are ubiquitous and seem to interact with most GPCRs and other signaling molecules. How so few arrestin and GRK isoforms manage to specifically regulate signaling via so many diverse GPCRs is one of the most intriguing unanswered questions in cell biology.