Welcome to the Kaverina Lab Website!
Welcome to the Kaverina Lab Website! 
We are actively recruiting graduate students and postdoctoral fellows. Check out the ‘Join us’ tab!
Research Focus:
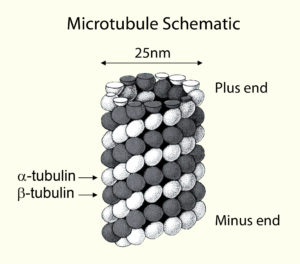
Microtubules (MTs), 25-nm self-assembling polymers, serve as highways for organelles and molecular transport within a cell. During cell division, MTs are arranged into the mitotic spindle and drive chromosome segregation. In interphase cells, MT network organization and modes of MT-dependent transport are much more variable, reflecting functional diversity of cells and tissues. For example, MT functions include secretory trafficking, organelle positioning, and control of site-specific activities (e.g. actin polymerization), thereby defining cell shape and physiology. Paradoxically, our understanding of interphase MT networks is by far less advanced that the understanding of mitotic machinery. The Kaverina Lab at Vanderbilt aims to close this gap in knowledge. We study how complex MT networks are arranged, and how specialized MT arrays support distinct cellular tasks.
Our lab is interested in:
- MT functions in cell architecture, processes, and physiology
MTs are important in a number of cellular processes, including cell division and intracellular transport. We are currently investigating the role of MTs in 1) Golgi organization in interphase, and 2) microtubule-associated proteins (MAPs) in intracellular transport.
- MT organization and functions in pancreatic beta cells
We are particularly interested in the role that MTs play in pancreatic beta cells and diabetes. MTs have long been known to transport insulin-containing granules to the membrane, however, our lab has found that MTs play a stronger role in preventing over-secretion of insulin to maintain normal glucose homeostasis (cite). We are currently interested in 1) paracrine/alpha cell-beta cell signaling, 2) MT sliding, 3) insulin secreting adhesions, and 4) mechanobiology of insulin secretion.
- MTs in other processes and diseases
Our interest in MTs extends beyond what you see here- if you are interested about their role in other systems such as immunology, cancer, etc., we are always excited to broaden our horizons!
Visit our “Research Projects” pages to read more about these ongoing projects and others that are open!
Our lab philosophy:
The astrophysicist Bernard Haisch once said
“Advances are made by answering questions. Discoveries are made by questioning answers.”
This idea is very appealing to us: we want to make discoveries! We base our research on dogma-challenging hypotheses that we test through a unique combination of cutting edge high-resolution microscopy techniques, supported by molecular approaches and merged with physiological assays and mathematical modeling.
EB comets (green) and Golgi (red)