Additional Projects
There are also a number of smaller projects currently under investigation:
-
The Role of MTs in Lithium Nephrotoxicity
Lithium compounds are the most common and powerful therapeutic treatment for bipolar disease. Unfortunately, such treatment is associated with severe side effects on the kidney. Distinguishing lithium therapeutic effects from its side effects will improve its capacity to treat bipolar disease. We have identified the microtubule (MT) cytoskeleton as one of the major targets of lithium signaling in kidney epithelium. We are currently focusing on understanding the role of MTs in lithium nephrotoxicity at the cellular level using MDCK cells as a model system. -
MT-dependent Regulation of the Actin Cytoskeleton
Many features of the actin cytoskeleton are regulated by MTs and MT-binding proteins. One major function of smooth muscle cells, contraction, is accomplished by acto-myosin stress fibers. We are now investigating the role of MT-binding proteins in stress fiber organization and contractile properties of smooth muscle cells in collaboration with Tatyana Svitkina’s lab at University of Pennsylvania. -
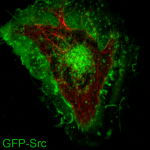
The Function of Cytokeletal Crosstalk in Vesicle Trafficking
Cytoskeletal crosstalk is a cellular phenomenon in which MTs and actin interact for the efficient coordination of a process. Src, a proto-oncogene, is thought to be trafficked from the cell center to the cell periphery in a mechanism that is reliant on both cytoskeletal networks; however, the molecular mechanism of how this coordination occurs has yet to be determined. The goal of this project is to elucidate the molecular players involved in the efficient delivery of Src, so potential targets for cancer therapies can be identified. -
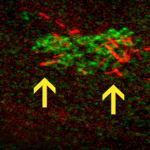
RASSF1A function in MT Network Dynamics and Golgi Positioning
Ras-association domain family 1A (RASSF1A) is a MT binding protein that is frequently silenced during cancer transformation. This loss of RASSF1A expression often correlates with increased levels of metastasis; however, the cell biological function of RASSF1A binding to MTs, or how loss of RASSF1A influences the MT network of interphase cells, has not been explored. The goal of this project is to utilize a variety of high-resolution live-cell imaging techniques to examine the effect that RASSF1A has on MT network organization and dynamics as well as its influence on other organelles in the cell; in addition, we are examining how these functions of RASSF1A subsequently affect MT-based processes, such as cell migration and polarity (Molecular Biology of the Cell 2014). This project is in collaboration with Geoff Clark’s lab at the University of Louisville.