Synaptic Remodeling
Synaptic remodeling is evolutionarily conserved
Neurons adopt polarized morphologies to direct the flow of information in the nervous system. The assembly of specialized presynaptic and postsynaptic regions is necessary for the transmission of signals from one neuron to the next. These domains may be remodeled during development or in learning and memory mechanisms. The molecular pathways that govern these events are poorly understood but the evident conservation of this phenomenon among diverse species suggests that studies in simple model organisms could reveal fundamental elements of synaptic remodeling. The GABAergic motor neurons in the nematode C. elegans display a striking example of developmentally regulated synaptic remodeling. Dorsal D (DD) motor neurons initially innervate ventral muscles but switch polarity to synapse with dorsal muscle during the first larval (L1) stage (White et al., 1978). Ventral D (VD) motor neurons are generated during the L1 stage and adopt the ventral polarity previously assumed by DD motor neurons. Thus, the polarity reversal of DD motor neurons insures that both dorsal (DD) and ventral (VD) muscles receive GABAergic inputs. We are exploiting this system to uncover genetic factors that govern motor neuron remodeling. These results could lead to significant advances in our understanding of synaptic plasticity and thereby provide a foundation for developing therapeutic approaches for human diseases that disrupt synaptic assembly.
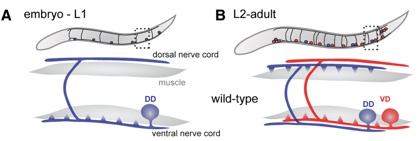
Figure 1. DD neurons remodel in the first larval (L1) stage. DD neurons switch synaptic output from ventral to dorsal muscles (blue triangles) whereas postembryonically derived VD neurons innervate ventral muscles (red triangles) and do not remodel.
UNC-55 functions as a transcriptional switch to prevent synaptic remodeling
VD motor neurons are prevented from remodeling by the nuclear hormone receptor UNC-55, the C. elegans homologue of COUP-TFII transcription factors (Walthall and Plunkett, 1995, Shan et al, 2005). In unc-55 mutants, VDs adopt the dorsal axonal polarity normally reserved for mature DDs. Thus, UNC-55 functions as a binary switch in VD motor neurons to block a synaptic remodeling program that results in dorsally directed axonal output. Because UNC-55 is likely to function as a transcriptional repressor, this mechanism is predicted to require UNC-55-dependent repression of synaptic remodeling genes in VD motor neurons.
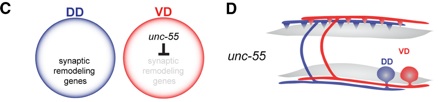
Figure 2. The COUP/TF transcription factor, UNC-55, is selectively expressed in VD neurons to block the synaptic remodeling program. (D) In unc-55 mutants, VD neurons switch ventral outputs to the dorsal side and thus adopt the synaptic polarity of DD neurons.
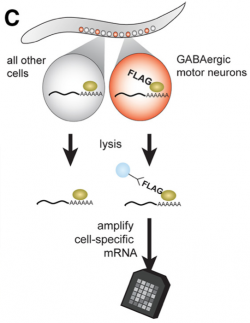
Figure 3. Strategy for identifying UNC-55 target genes. The mRNA tagging strategy was used to collect transcripts from GABAergic motor neurons. A comparison of wild-type vs unc-55 microarray profiles identified unc-55-regulated transcripts.
Expression profiling reveals candidate synaptic remodeling genes
We devised a cell-specific microarray profiling strategy to identify UNC-55 target genes (Petersen et al, 2011). Our approach is based on the assumption that UNC-55-regulated transcripts should be up-regulated (i.e., de-repressed) in unc-55 mutant VD motor neurons. To identify these UNC-55-regulated transcripts, we used the mRNA tagging strategy (Roy et al., 2002; Von Stetina et al., 2007; Watson, et. al., 2008) to compare microarray profiles of wild-type vs unc-55 mutant GABAergic motor neurons. Because UNC-55 is predicted to function as a repressor, we examined genes that were significantly enriched in unc-55 GABA motor neurons.
A transcriptional program remodels GABAergic synapses.
We used the synaptic marker, SNB-1::GFP to mark the axonal domains of DD and VD GABAerigic motor neurons (Hallam and Jin, 1998). In unc-55 mutant adults, SNB-1::GFP puncta are largely limited to the dorsal nerve cord due to ectopic remodeling of VD motor neurons (Fig 1). We reasoned that the absence of ventral SNB-1::GFP puncta should depend on UNC-55 targets and therefore that RNAi of these genes should “suppress” this effect (i.e., restore SNB-1::GFP puncta to the ventral side). RNAi knockdown of candidate UNC-55-regulated transcripts identified in the microarray experiment revealed 50 genes that at least partially suppressed the Unc-55 remodeling defect (p<0.01). Most striking among these candidates is the homeobox transcription factor IRX-1/Iroquois, which suppresses both the loss of ventral SNB-1::GFP puncta and the Unc-55 backward movement defect . Other suppressors of the Unc-55 synaptic remodeling phenotype include specific ion channels (see below), cytoskeletal components, and cell-cell signaling molecules. The wide range of potential functions encoded by these UNC-55-regulated genes is indicative of a complex synaptic remodeling pathway. Ongoing experiments are designed to confirm that these genes function in the unc-55 pathway and to define their cellular roles in the mechanism of synaptic remodeling (Petersen et al, 2011).
The DEG/ENaC cation channel protein UNC-8 drives activity-dependent synapse removal in remodeling GABAergic neurons.
In recent work, we confirmed that the UNC-55 regulated target, the DEG/ENaC cation channel protein, UNC-8, drives the removal of ventral presynaptic domains in remodeling GABAergic neurons (Miller-Fleming, Petersen et al,, 2016)
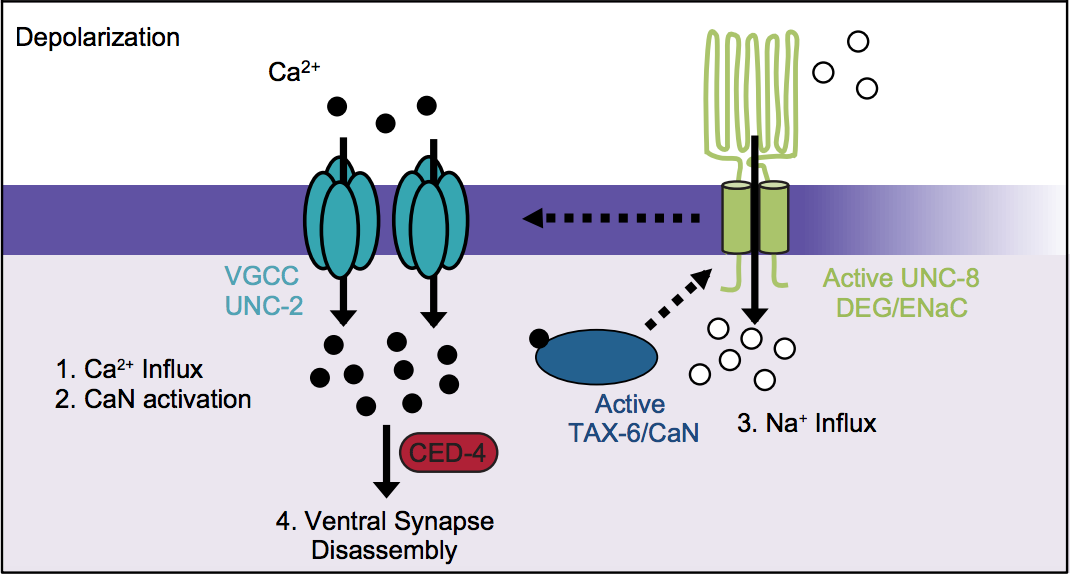
Figure 4 Proposed mechanism of UNC-8-driven synapse disassembly in remodeling GABA neurons. UNC-8 drives synaptic removal by triggering a positive feedback loop that elevates intracellular calcium to activate a caspase-dependent mechanism that destroys the presynaptic domain. GABA neuron depolarization results in Ca++ import by UNC-2/VGCC. Activation of the calcium-dependent phosphatase TAX-6/Calcineurin is proposed to stimulate UNC-8-dependent Na+ import which further depolarizes the presynaptic membrane to enhance Ca++ by UNC-2/VGCC.