MSTPublications: May 2018
Congratulations to all of our MSTP students on their successful publications! Take a look at the great work our students are doing.
First Author Original Research:

Heterozygous loss of TSC2 alters p53 signaling and human stem cell reprogramming.
Armstrong LC, Westlake G, Snow JP, Cawthon B, Armour E, Bowman AB, Ess KC. Hum Mol Genet. 2017 Dec 1;26(23):4629-4641. doi: 10.1093/hmg/ddx345.
Tuberous sclerosis complex (TSC) is a pediatric disorder of dysregulated growth and differentiation caused by loss of function mutations in either the TSC1 or TSC2 genes, which regulate mTOR kinase activity. To study aberrations of early development in TSC, we generated induced pluripotent stem cells using dermal fibroblasts obtained from patients with TSC. During validation, we found that stem cells generated from TSC patients had a very high rate of integration of the reprogramming plasmid containing a shRNA against TP53. We also found that loss of one allele of TSC2 in human fibroblasts is sufficient to increase p53 levels and impair stem cell reprogramming. Increased p53 was also observed in TSC2heterozygous and homozygous mutant human stem cells, suggesting that the interactions between TSC2 and p53 are consistent across cell types and gene dosage. These results support important contributions of TSC2 heterozygous and homozygous mutant cells to the pathogenesis of TSC and the important role of p53 during reprogramming.
Association of ST2 polymorphisms with atopy, asthma and leukemia.
Bloodworth MH*, Rusznak M*, Bastarache L, Wang J, Denny JC, Peebles RS Jr.
J Allergy Clin Immunol. 2018 May 19. pii: S0091-6749(18)30712-7. doi: 10.1016/j.jaci.2018.03.020. [Epub ahead of print]
IL-33 is one of the most consistently associated gene candidates for asthma identified by GWASs in diverse ethnic groups. IL-33 is a central mediator of both innate and adaptive immunity- regulated allergic inflammation in the lung. ST2 is the primary binding partner of IL-33. We performed a phenome-wide association study (PheWAS) to assess the relationship between single nucleotide polymorphisms (SNPs) in the ST2 gene and various disease phenotypes. The PheWAS is a new, validated reverse genetics approach that associates genetic variants of interest with phenotypes by linking a database of de-identified genotyping to a broad range of electronic medial record (EMR)-derived clinical phenotypes. The primary significance of our work is three-fold. Firstly, our study identifies multiple SNPs in ST2 that correlate with allergic conjunctivitis and eosinophilia. These SNPs were discovered with GWASs in the context of asthma, and their genetic connection to these other allergic diseases helps to elucidate the mechanisms of their pathogenesis. Secondly, the study confirms the previously reported associations between asthma and ST2 signaling. Finally, we implicate ST2 signaling in leukemia at the genetic level by finding significant associations between ST2 SNPs and leukemia diagnoses. These results not only corroborate preliminary in vivo studies investigating the role of the IL-33/ST2 signaling pathway in leukemia, but they also demonstrate the utility of PheWAS in identifying genetic connections to diseases not previously considered. Mark Rusznak, an exceptionally talented, freshly minted graduate of Pomona College and a Nashville native, is a co-first author on this study. He has had a highly productive two summers in the Peebles lab, will continue to work in the lab over the next year, and will be applying to MD/ PhD programs this fall! (By Melissa Bloodworth, M3)
MFehi adipose tissue macrophages compensate for tissue iron perturbations in mice.
Hubler MJ, Erikson KM, Kennedy AJ, Hasty AH.
Am J Physiol Cell Physiol. 2018 May 16. doi: 10.1152/ajpcell.00103.2018. [Epub ahead of print]
Adipocytes can be sensitive to excess iron because their lipid contents make the susceptible to oxidative stress. In these studies we go into depth to explore how adipose-tissue macrophages protect adipocytes from iron-overload. We approach this with two methods; the first is a model of chronic dietary-iron excess, the second acute iron injections. In both cases, we observed that macrophages in the tissue take up excess iron, adjust their gene expression, and prevent adipocytes from taking up iron. We also are the first to describe that excess iron is able to induce recruitment of monocyte to adipose tissue. Furthermore, iron injections lead to a shift towards anti-inflammatory polarization of the macrophages that took up the iron. These studies shed light on the importance of resident macrophages in normal adipose tissue function and explore iron as another form or homeostasis and nutrient partitioning for which macrophages are responsible in this tissue. (By Merla Hubler, G5)
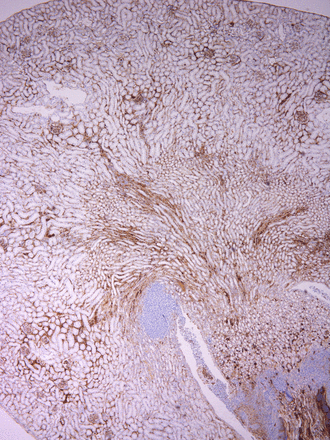 Work by Ben Fensterheim (M3) made the cover of the June 1 issue of The Journal of Immunology! This publication was highlighted in last month’s publications.
Work by Ben Fensterheim (M3) made the cover of the June 1 issue of The Journal of Immunology! This publication was highlighted in last month’s publications.
About the Cover Art: Histologic section of a kidney from a monophosphoryl lipid A–treated CCR2 knockout mouse 3 d after infection with Staphylococcus aureus. An F4/80 immuohistochemistry stain (brown) demonstrates that monophosphoryl lipid A prophylaxis boosts macrophage abundance in the kidney despite a lack of CCR2-dependent inflammatory monocytes.
Fensterheim, B. A., J. D. Young, L. Luan, R. R. Kleinbard, C. L. Stothers, N. K. Patil, A. G. McAtee-Pereira, Y. Guo, I. Trenary, A. Hernandez, J. B. Fults, D. L. Williams, E. R. Sherwood, and J. K. Bohannon. 2018. The TLR4 agonist monophosphoryl lipid A drives broad resistance to infection via dynamic reprogramming of macrophage metabolism. J. Immunol. 200: 3777–3789.
Co-authorships, Clinical Studies, and Reviews:
Human Monocyte Transciptional Profiling Identifies Interleukin 18 Receptor Accessory Protein and Lactoferrin as Novel Immune Targets in Hypertension.
Alexander MR, Norlander AE, Elijovich F, Atreya RV, Gaye A, Gnecco JS, Laffer CL, Galindo CL, Madhur MS.
Br J Pharmacol. 2018 May 18. doi: 10.1111/bph.14364. [Epub ahead of print]
A Customizable, Low-Cost Perfusion System for Sustaining Tissue Constructs.
O’Grady B, Wang J, Faley S, Balikov D, Lippmann E, Bellan LM.
SLAS Technol. 2018 May 1:2472630318775059. doi: 10.1177/2472630318775059. [Epub ahead of print]
Selective Packaging in Murine Coronavirus Promotes Virulence by Limiting Type I Interferon Responses.
Athmer J, Fehr AR, Grunewald ME, Qu W, Wheeler DL, Graepel KW, Channappanavar R, Sekine A, Aldabeeb DS, Gale M Jr., Denison MR, Perlman S.
MBio. 2018 May 1;9(3). pii: e00272-18. doi: 10.1128/mBio.00272-18.
Reovirus Nonstructural Protein σNS Acts as an RNA-Stability Factor Promoting Viral Genome Replication.
Zamora PF, Hu L, Knowlton JJ, Lahr RM, Moreno RA, Berman AJ, Prasad BVV, Dermody TS.
J Virol. 2018 May 16. pii: JVI.00563-18. doi: 10.1128/JVI.00563-18. [Epub ahead of print]
Want to be sure we don’t miss your MSTPublication? Email mdphd@vanderbilt.edu to tell us about it!