Cultivating hope
Jackie Hill had taught early childhood education at Chattanooga State Community College for 24 years when her students noticed something troubling.
She was giving them the same assignments repeatedly, or she’d give them the wrong assignment.
There were other indications, too, said her longtime friend and life partner Ivan O’Neal.
In 2011, Hill traveled with a friend, and when they returned, the friend told O’Neal that Hill should see a physician and be evaluated for dementia.
Shortly after, Hill was diagnosed with early onset Alzheimer’s disease. She was only 56.
“It’s so strange, the people closest to them, we go into denial too,” O’Neal said. “They don’t want to accept it, and we don’t want to believe it.”

Hill, now 65, is one of hundreds of patients diagnosed with Alzheimer’s disease and other forms of dementia who are enrolled in studies through the Vanderbilt Memory and Alzheimer’s Center (VMAC), which transitioned into a freestanding institutional center in 2020. The center’s director is Angela Jefferson, PhD, professor of Neurology and Medicine, an internationally recognized researcher in cardiovascular and cerebrovascular contributions to Alzheimer’s disease and related dementias (ADRD).
Hill is participating in a clinical trial investigating the drug troriluzole to treat mild to moderate Alzheimer’s disease. Vanderbilt is one of several sites across the United States enrolling patients in the trial, and Jefferson is Vanderbilt’s site investigator.
The study is designed to determine if the drug can protect against, slow down and potentially improve memory problems that increase as Alzheimer’s disease progresses. Neither Hill nor Jefferson’s team knows if she is receiving the drug or a placebo.
Although the funding for research into Alzheimer’s disease has increased each year since President Obama enacted the National Alzheimer’s Project Act (NAPA) in 2011, the U.S. Food and Drug Administration (FDA) has not approved a new medication for its treatment since 2003. The FDA will be making an approval decision on Biogen’s aducanumab this year. If approved, aducanumab will be the first new disease-modifying Alzheimer’s treatment. All previously-approved drugs modestly treat symptoms rather than modify disease progress.
By comparison, the National Institutes of Health (NIH) allocated $2.5 billion in 2019 to domestic research into HIV, which affects 1.2 million people. The same year, NIH allocated $2.8 billion to Alzheimer’s disease and related dementia research, which affects 5.2 million people – so Alzheimer’s disease received 12% more funding but affects four times the people.
“As part of NAPA, the federal government is investing more and more money in Alzheimer’s research. Over the last five years, we have seen federal funding allocated to ADRD research increase by a factor of four, yet only one in four applications is funded,” Jefferson said. “We have found that philanthropic partnerships are vital in helping us generate strong pilot data to increase the likelihood of securing an NIH award on our first submission.”
Vanderbilt received two major grant awards to support Alzheimer’s disease research last year. In June 2020, the Vanderbilt Memory and Aging Project received an $18.2 million, five-year grant renewal from the National Institute on Aging (NIA). Founded by Jefferson in 2012, this interdisciplinary project at Vanderbilt is gathering comprehensive longitudinal cardiovascular and cerebrovascular health measures on hundreds of adults in Middle Tennessee to identify risk factors and vascular contributions to ADRD.
In August 2020, Jefferson and her colleagues were awarded a $3.7 million, three-year grant from the NIA to establish a prospective NIA-funded Alzheimer’s Disease Research Center at Vanderbilt. The award is intended to support planning and infrastructure development for a larger NIA research center application and NIH Center of Excellence designation for Alzheimer’s efforts at Vanderbilt University Medical Center.
The VMAC brings together Vanderbilt University and VUMC disciplines such as neurology, psychiatry, medicine, radiology, health policy, pharmacology and pathology — all focused on clinical, research and training initiatives geared toward the daunting task of finding treatments or a cure for Alzheimer’s disease. Prior to becoming a freestanding center, the VMAC was housed in the Department of Neurology.
“As the elderly population in the United States continues to grow, Alzheimer’s disease and related dementias have become an important strategic area for investment and growth across VUMC’s research and clinical enterprises,” said Jennifer Pietenpol, PhD, Executive Vice President for Research. “ADRD remains the only leading cause of death in the United States without effective prevention or therapeutic interventions. As these illnesses threaten to affect yet greater numbers of patients and families, this new center will help place VUMC at the leading edge of ADRD discovery, care and education.”
Prevention or a cure?
Jefferson said she’s hopeful that Alzheimer’s disease, like HIV, will morph from a devastating diagnosis to a treatable chronic condition, enabling those diagnosed to live longer, healthier lives.
“I think a true cure will probably never exist given the complexity of the disease, but I’m still hopeful we will figure out how to identify and treat the disease in patients before memory loss symptoms appear and affect their everyday lives,” Jefferson said.
Adding to the challenges of Alzheimer’s research is the complexity of the human brain.
“The brain is the most complex structure in the known universe,” said Paul Newhouse, MD, professor of Psychiatry and Pharmacology, Jim Turner Professor of Cognitive Disorders and VMAC investigator. “We are learning a whole lot, but there is much to learn. We have had successes, but also a lot of failure, and we have to be comfortable with that. We have to keep plugging away. I remain optimistic we’ll make some real progress, but this is a very biologically difficult disease.”

The healthy human brain contains tens of billions of neurons that process and transmit information via electrical and chemical signals. They send messages between different parts of the brain, and the brain sends the messages to the body’s muscles and organs. Alzheimer’s disease disrupts this communication, resulting in loss of function and cell death.
Until now, most Alzheimer’s research has focused on the two primary targets for treatment — beta-amyloid plaques and tau tangles, buildups of proteins that damage brain tissue. Sticky beta-amyloid protein fragments accumulate in spaces between nerve cells, and the twisted forms of tau crowd inside brain cells.
These proteins occur as part of the normal aging process, but in people with Alzheimer’s and related dementias, the amounts of these proteins that build up are far greater, and the forms of the proteins are different. They exist in the brain in their non-toxic, non-diseased form, but something goes haywire causing both proteins to form pathological versions that accumulate and cause damage to the surrounding cells.
Investigators at Vanderbilt are pioneering new approaches based on novel pathways that intersect with these abnormal proteins, searching for ways to reduce risk and offer protection against the disease.
“For many years, much of the field has been pursuing questions along the same hypothesis,” Jefferson said. “Amyloid is definitely an essential component of the pathological diagnosis of the disease, but there is much more to Alzheimer’s disease than just amyloid accumulation. Our overemphasis on amyloid has come at the expense of innovation and discovery, in my opinion. We have put so much effort into chasing the amyloid hypothesis, yet every single amyloid-focused trial has come up short. At some point we need to think outside the box. With more funding opportunities, there are new hypotheses and more sensitive technologies emerging. I am optimistic we are on the cusp of significant breakthroughs.”
Other pathologies, like tau tangles, correlate more strongly with the clinical symptoms of Alzheimer’s disease, she said. A study in 2020, for example, showed that brain imaging of tau protein tangles predicts the location of future brain atrophy in Alzheimer’s patients a year or more in advance. In contrast, the location of amyloid plaques was found to be of little use in predicting how the brain would be damaged as the disease progressed.
“The reality is that the pathology underlying ADRD unfolds about two decades or more before someone manifests any memory loss symptoms. It is scary to think that the disease is unfurling without our knowledge,” Jefferson said.
Although Alzheimer’s disease is the most common form of ADRD, other forms include Lewy body dementia (comedian and actor Robin Williams was diagnosed with this before his death in 2014), vascular cognitive impairment and dementia, and frontotemporal dementia.
Those with Alzheimer’s disease rarely present with one cleanly defined pathology, and can in fact have multiple forms of overlapping dementia. For example, a 2017 study showed that nearly 9 out of 10 people with Alzheimer’s disease at autopsy also have some other form of vascular disease in their brain, including atherosclerosis and arteriosclerosis.
“At VMAC, we’re really interested in the idea of concomitant pathologies — that there are other processes in the brain that either just exist as a neighbor next to Alzheimer’s and more pathology just means more symptoms, or they actually intersect in some meaningful way with Alzheimer’s to accelerate symptoms, manifestation and clinical progression,” Jefferson said. “These concomitant pathologies are a major focus of the recent renewal of the Vanderbilt Memory and Aging Project grant.”
When cerebrovascular disease coexists with Alzheimer’s disease, those people may manifest memory loss and cognitive symptoms earlier than peers with just Alzheimer’s disease and they get worse at a much faster rate, Jefferson said.
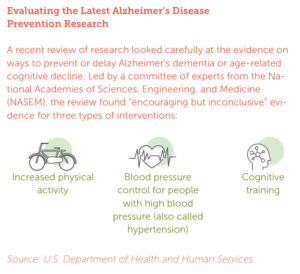
“Interestingly, the things that drive vascular disease in the brain are all the things that cause cardiovascular disease — high blood pressure, high cholesterol, smoking, diabetes, and lack of physical activity,” Jefferson said. “Each of these risk factors is preventable or treatable through already approved medications and lifestyle changes. The ability to prevent vascular disease in the brain by properly managing these risks has potentially enormous benefits and impact.”
A 2019 study in the Journal of the American Medical Association showed that more aggressive management of blood pressure resulted in fewer new cases of early dementia, known as mild cognitive impairment (MCI), over time.
“One major area of our research focus in the Vanderbilt Memory and Aging Project is how vascular stiffness contributes to ADRD. Blood vessels should be malleable like a garden hose, but the longer you live, the more they lose that flexibility and behave more like a lead pipe. That increasing stiffness is what drives higher blood pressure with age. We have linked increasing stiffness to very early blood flow changes in the exact same areas of the brain where Alzheimer’s disease first evolves,” Jefferson said.
“It is possible that age-related changes in the cardiovascular system increase the vulnerability of certain brain regions to ADRD pathology. Over the next few years, we hope data from the Vanderbilt Memory and Aging Project will help us answer this.”
Similar to some adults living with hypertension, Jefferson said she believes people with Alzheimer’s disease will someday prevent or manage their disease by taking a combination of medications.
“I don’t think the solution will be one single drug. I think prevention or treatment will be a cocktail of medications that target different risk factors or drivers of pathology,” Jefferson said.
Sex differences
Almost two-thirds of Americans with Alzheimer’s are women.
There is mounting evidence that the higher prevalence of Alzheimer’s disease among women may not simply be a consequence of a longer lifespan for women.
There may be multiple factors at play, Newhouse said, including the loss of hormones that support brain function at menopause.
The contribution of menopause is being studied by VMAC investigators through the NIA-funded CHAMP study (Cognitive Health After Menopause). The study looks at identifying biological markers of Alzheimer’s disease risk in women 10 to 20 years after menopause. “We think if we can identify a subgroup of women at risk, that will allow us to design prevention or other strategies to lower their risk,” Newhouse said.
Another theory is that women’s brains may spread the abnormal tau proteins connected with Alzheimer’s disease more effectively. That theory is also being studied by VMAC investigators.
“It may be that women have better internal connectivity in their brain, which may explain their superior abilities in things like verbal memory, but that better connectivity may increase their risk of any abnormalities spreading along these structural networks. It may be that tau has a better railway to spread on, if you will,” Newhouse said.
Vanderbilt has also developed a positron emission tomography (PET) imaging agent — a tracer that will allow researchers for the first time to get real-time information about the integrity of certain brain systems that are important for learning and memory. VMAC investigators collaborating with the Vanderbilt University Institute of Imaging Science developed the new tracer, FFEOBV, a promising marker for the detection of alterations in the brain’s cholinergic system, involved in the regulation of attention and higher-order cognitive processing.
In collaboration with the University of Vermont, Vanderbilt investigators will recruit more than 120 postmenopausal women ages 50 to 70 over the next several years to look at amyloid in their brains. The study will look at the pathology within the brain in many different ways including sampling cerebrospinal fluid and using blood-based biomarkers. “We think women may fall into a grade of risk — high to low — and we want to be able to identify one of the earliest signs of biological changes in the brain,” Newhouse said.
The brain’s cholinergic system is also the target of the new molecule (VU319) developed by the Warren Center for Neuroscience Drug Discovery (see story on page 19) in collaboration with Newhouse and colleagues at the Center for Cognitive Medicine. This potential Alzheimer’s disease treatment recently completed its first-in-human
trials at Vanderbilt under Newhouse’s direction and has been licensed to a pharmaceutical company for further development.
Newhouse is also directing a national multicenter NIA-funded trial examining transdermal nicotine to improve the cognitive symptoms (memory, attention) of patients with mild cognitive impairment (MCI), the precursor condition to Alzheimer’s disease/dementia. There are 42 sites around the country participating in the study.
And VMAC research is looking into the apolipoprotein E (APOE) gene, the strongest genetic risk factor for Alzheimer’s disease, showing that it may play a more prominent role in disease development among women than men.
A study published by VMAC investigators in 2019 confirmed recent studies that carrying the APOE ε4 allele has a greater association with Alzheimer’s disease among women compared to men and went one step further by evaluating its association with amyloid and tau levels.
The research, based on a meta-analysis of both cerebral spinal fluid (CSF) samples from study volunteers from four datasets and autopsy findings from six datasets of Alzheimer-diseased brains, is the most robust evidence to date that the APOE gene may play a greater role in women than men in developing Alzheimer’s pathology, said Timothy Hohman, PhD, associate professor of Neurology and the study’s lead author.
“In Alzheimer’s disease, we have not done enough to evaluate whether or not sex is a contributing factor to the neuropathology,” Hohman said. “We haven’t fully evaluated sex as a biological variable. But there is good reason to expect in older adulthood that there would be hormonal differences between the sexes that could impact disease.”
The study looked at whether APOE in men and women was primarily associated with the amyloid or with the tau pathway.
The association with the amyloid pathway was the same in men and women. However, the APOE association was much greater for women with the tau pathway. This is opposite of what researchers expected because of APOE’s established role in amyloid processing.
In 2020, Hohman’s team extended this work and identified multiple genes that act in only women or only men to drive amyloid and tau pathology. All of the sex differences research at Vanderbilt is building the foundation for precision medicine, where the best treatment target can be selected for the individual patient. The ideal target for a woman may be quite different than the ideal target for a man.
Racial and ethnic disparities
African Americans, like Hill, are about twice as likely and Hispanic and Latinos 1.5 times as likely to be diagnosed with Alzheimer’s disease and other dementias than whites, said Consuelo Wilkins, MD, MSCI, professor of Medicine and Vice President for Health Equity at VUMC. And they are more likely to have worse outcomes.
Wilkins is guiding the VMAC’s efforts to increase the number of racial and ethnic minorities enrolled in the center’s research studies.
“There’s a long history of fewer minorities participating in research about a condition that is overrepresented among them,” Wilkins said. “Until the last few decades most research in general was done on middle-aged white men; it wasn’t until 35 years ago that women in general started to be included, and there was also an age limit on many studies with the highest age being 55 — that’s slowly been increasing, which is important in studying a condition that’s more common in older people.”

There are theories about why a higher number of African Americans, Latinos and Hispanics are diagnosed with Alzheimer’s disease and why they have worse outcomes.
“It might be related to vascular disease — there’s a higher proportion of comorbidities among these populations — and there are also societal issues, concerns about the environment and interactions in the environment that could be increasing the risk of the disease,” Wilkins said. “There may also be biases in how people present and how they’re screened and the kinds of tests they have access to — many in these populations are less likely to see a dementia specialist.”
There has also historically been a distrust of research among African Americans, dating back to the Tuskegee Study of Untreated Syphilis that involved 600 Black men, conducted without the patients’ informed consent, Wilkins said.
Wilkins is co-leading recruitment for a national study in collaboration with the University of North Carolina Chapel Hill that will include 4,000 minorities — 2,000 African Americans and 2,000 Hispanics — who have symptoms of ADRD. The study will use brain PET imaging to study amyloid plaque as a marker for ADRD. A previous large study using PET imaging included 18,500 Medicare beneficiaries, but less than 10% were minorities, she said.
Screening for dementia also needs to be addressed in racial and ethnic minority groups, she said. For example, a common memory test has the word “coriander” in it. “If you’re from a culture or a population or socioeconomic class that might not be familiar with coriander, or if the first time you hear the word coriander is when someone is testing your memory, it’s less likely you’ll be able to recall that word,” Wilkins said.
O’Neal, from Chattanooga, remembers Hill’s screening well. She was seen at VUMC in 2011 by Howard Kirshner, MD, professor of Neurology and VMAC faculty member.
Hill, a longtime educator who had earned her PhD in early childhood education only two years before, was given three words during her examination and told she’d be asked to recall them. She could not. Then Kirshner gave her a sheet of paper with an empty clock face and asked her to set the clock to read 10 past 11 o’clock.
 “When I saw that she couldn’t figure that time out, I stepped out of the room and that’s when I cried. I got it out then and haven’t cried since. I have to be strong,” O’Neal said.
“When I saw that she couldn’t figure that time out, I stepped out of the room and that’s when I cried. I got it out then and haven’t cried since. I have to be strong,” O’Neal said.
Early diagnosis is important, Vanderbilt’s Jefferson says.
“You want to give people optimism and hope at the time of diagnosis, but the reality is right now we don’t have an effective therapy,” she said.
During a recent phone conversation, Hill struggled to retrieve words. When asked where she lives, she answers: “In Chattanooga. In a house. I can’t tell you the house number, but I know where my house is.”
O’Neal, whose mother also had dementia, said keeping Hill safe, listening to what she has to say, and making her laugh are his priorities.
“She was my right hand when my mother passed, and as soon as I brought my mother down those church steps, then Jackie got it. I learned with my mother to listen as much as you can, instead of always trying to correct them. I’ve been there with her since the beginning. It’s not going to turn around, so I just want to continue to make her happy. She’s in a good space about it right now.”
Hill understands that she has dementia, but her bubbly personality is evident, and she laughs often during conversations.
“Baby girl, I’m still cute, walking and talking, but I can’t remember nothing,” she said during the recent conversation. “I can’t change what is happening, but you just have to deal with it.”
