
Shaping the Future of Neuroscience
Faculty, students and staff across campus are exploring everything from addiction, anxiety, sleep and memory; to sensory and motor functions, neuroendocrine signaling and neuroengineering.
From molecules to the mind, our graduate students and faculty are involved in translational and clinical research that encompasses nearly all of contemporary neuroscience.
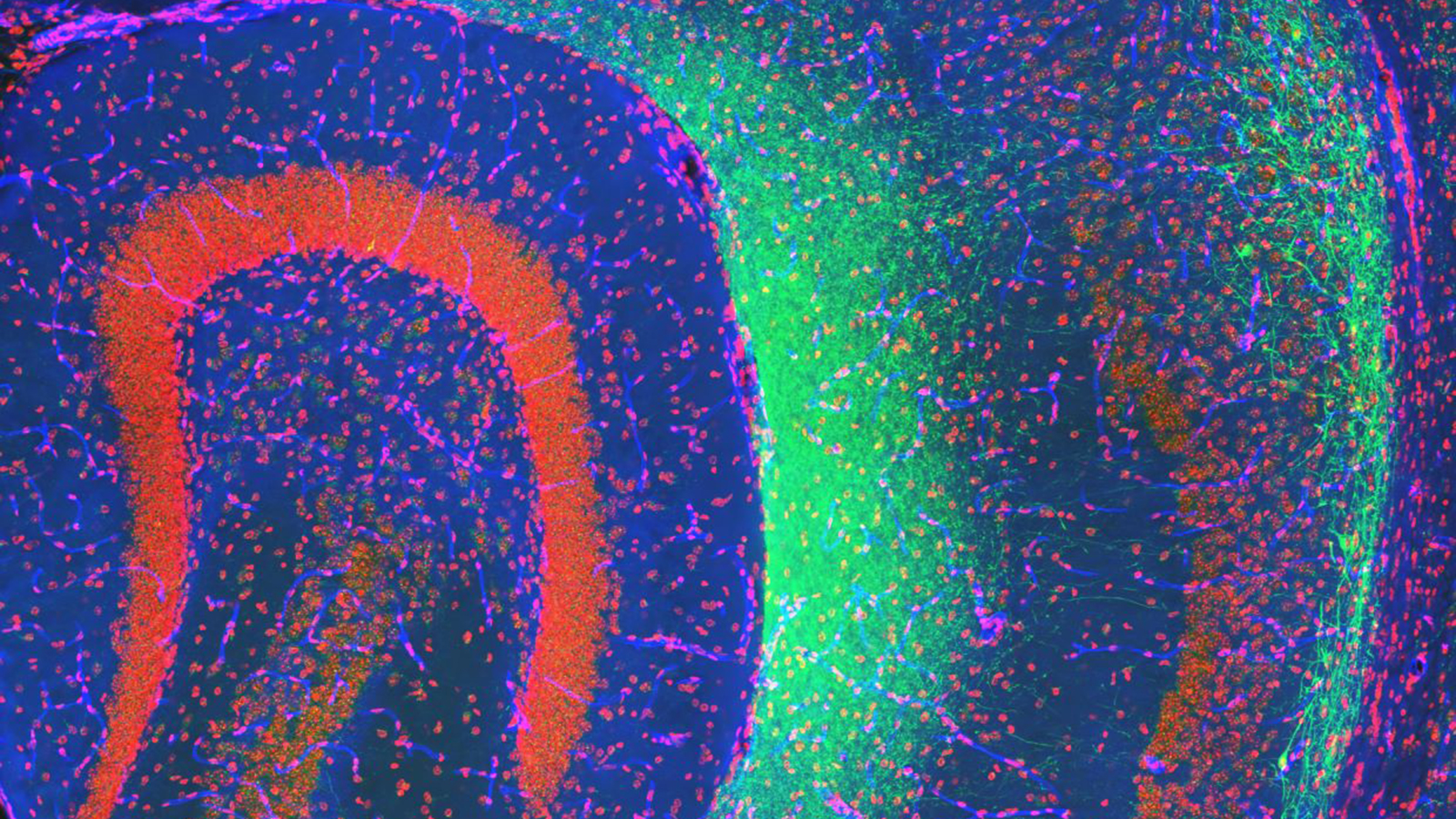

Faculty, students and staff across campus are exploring everything from addiction, anxiety, sleep and memory; to sensory and motor functions, neuroendocrine signaling and neuroengineering.
Access to our state-of-the-art laboratories, instruments and equipment empowers our VBI community to explore, experiment and fulfill their research goals.


My research focuses on the intersection of neuroimaging and computational genetics, and white matter microstructural decline in neurodegenerative disorders (e.g., Alzheimer's disease).
The development and application of novel imaging methods for the study of central nervous system development and function in health and disease.
Research in the Ayala lab focuses on gut-brain interactions that regulate energy balance and feeding behavior.
How is it that we attend to important stimuli in our environment? The mission of the Bastos Lab is to understand the neuronal basis of attention, predictive processing, and working memory.
Developing and applying computational methodologies to further our understanding of the genetic basis of human disease.
Dr. Belzil's laboratory is dedicated to utilizing human biospecimens to identify disease-specific variants, including genetic, epigenetic, and transcriptional changes.
My lab uses neurophysiology techniques in human subjects to study neural signaling underlying cognitive and psychiatric processes.
Pathogenesis and treatment of autism, drug development in autism; abnormal repetitive behaviors in neurodevelopmental disorders.
We are broadly interested in how the brain changes over development and learning, and in individual differences, including disability, in brain organization.
Nervous system development, including neuronal pathfinding, target recognition and synaptogenesis.
Our research seeks to characterize and modulate the precise circuits in the brain that underlie both adaptive and maladaptive processes in reward, motivation, and associative learning, to develop improved treatments for psychiatric disorders.
Mechanisms of neurodegeneration and molecular architecture of visual pathways in health and disease.
Determining signaling mechanisms which when abnormal, lead to neurodevelopmental defects, epilepsy, white-matter disorders, and autism.
Development of the peripheral nervous system; mechanisms of neurotrophin signaling, neuronal apoptosis, corpse clearance and glial-neuron interactions.
Neural basis of sensory processing differences in children and adults with autism using a combination of sensory testing and neuroimaging to investigate how different kinds of sensory processing are disrupted in autism.
The neurobiology and behavior of mammals, specifically pertaining the organization and function of mammalian sensory systems.
Increasing understanding of the human brain by advancing functional neuroimaging methodology.
My research interests are dedicated to understanding basic mechanisms of neuropsychiatric disease and applying that knowledge to develop new treatments.
Our research is dedicated to finding methods to regenerate the injured spinal cord, with the ultimate goal of reversing paralysis.
Brain-behavior relationships and biomarker identification using multimodal neuroimaging, biofluid analysis, and cognitive testing in patients with neurodegenerative disorders.
The regulation and roles of calcium/calmodulin-dependent protein kinase II (CaMKII).
How neuronal activity in the cerebral cortex gives rise to cognitive functions.
Examination of the Biobehavioral Profiles of Autism to Better Understand Factors That Enhance or Diminish the Response to Social and Nonsocial Stress.
Current research is focused on large-scale integration of genomic with other "-omics" data as well as biobank and electronic medical records data.
Understanding brain-behavior relations as related to learning and communication in children and adolescents.
Network neuroimaging and behavioral testing in patients with delusions, hallucinations, and criminal behavior due to dementia.
Molecular Physiology of CNS and PNS Cation-Chloride Cotransporters.
Dopamine and its Role in Neuropsychiatric Disorders.
Imaging biomarker development and clinical trials.
Developing cutting-edge molecular tools to uncover the complex interplay between neural signaling and behavior in health and disease.
Examination of the role of hippocampal-dependent memory in language and social interaction and in long-term outcome following acquired brain injury.
Neuronal injury and the role inflammation may play in brain aging and neurodegeneration.
Development and Correlates of Psychopathology and Behavioral Problems in Prader-Willi Syndrome, Williams Syndrome, and Down Syndrome.
Our lab focuses on the design and preclinical testing of experimental radiation treatment strategies that aim to reduce the severity of neurologic side effects for brain-cancer patients undergoing radiotherapy.
The research interests of our laboratory focus on the cellular and molecular processes underlying neuronal communication in normal and pathophysiologic disease states.
Our laboratory integrates human neuroimaging and electrophysiology techniques to study brain networks in both neurological diseases and normal brain states.
Regulation of eye development and regeneration.
Molecular, synaptic, and network changes in genetic models of human epilepsy syndromes.
Broad questions of how mitochondrial structure and apoptosis execution programs are coordinated to regulate cell fate, guide my laboratory research program.
Face and Object Recognition, Brain-Lesion Studies, and Visual Perception using Functional Imaging (fMRI).
Basic auditory function, spatial hearing, and speech perception with combined electric and acoustic hearing in adults and children with cochlear implants and hearing aids.
We specialize in combining microfabricated, transparent brain-machine interfaces with optical neurotechnologies for recording activity across massive spatial scales, from individual synapses up to brain-wide networks.
We investigate the role of genome integrity and the DNA damage response in Parkinson’s disease and other neurodegenerative diseases. Our research is multi-disciplinary, integrating cell biology, neuroscience, toxicology, and omics technologies.
The role of rhythm in language development using behavioral, EEG, and genomic methods.
Development and applications of imaging techniques for In-vivo studies of brain and spine.
Molecular mechanisms controlling the organization and composition of membranes important for vesicular transport and neuronal function.
Functional Organization of Reward Circuits and Mechanisms Underlying Motivational Learning.
My lab is studying fascinating proteins - G proteins-coupled receptor kinases (GRKs) and arrestins - and their role in regulating responsiveness of neurotransmitter receptors in the brain of living animals.
Transcriptomic profiling and cellular phenotyping of the auditory system.
The Structure, Function and Interactions of G-Protein Coupled Receptors; Molecular Mechanisms of Signal Transduction; Vascular Biology.
The roles of vitamin C in neuroprotection, neuromodulation and neuroinflammation in Alzheimer's disease and other models of cognitive decline.
Schizophrenia; Bipolar Disorder; Neuroimaging; Neuroanatomy.
The consequences of supply-limited brain metabolism for the putative limitation of lifespan by the number of neurons in the cerebral cortex.
Neural basis of learning and memory, large-scale electrophysiology, object and face recognition, neural oscillations, neural computation, sleep and state-dependent influences on perception.
Advanced computational approaches from genomics, proteomics, and neuroscience to identify novel markers of Alzheimer's disease risk and resilience.
Questions related to positive and negative experiences during pregnancy, infancy, and early childhood.
Studying neural stem cells, brain tumors, and the relationship between the two using high-dimensional approaches.
Molecular mechanisms of membrane trafficking proteins in cell biology and human brain disease.
Laser-tissue interaction; optical neural interfaces; modulation of neural activity using infrared laser light; cellular effects of laser-induced stimuli; application of light, lasers and optical technology in medicine and biology.
Vascular factors contributing to cognitive aging, Alzheimer's disease, cerebral small vessel disease and neurodegeneration using neuroimaging, cardiac imaging, neuropsychological testing, fluid biomarkers, and proteomics as well as translational animal and cellular models.
Cellular and molecular biology of biological clocks.
Law and Behavioral Biology; Law and Neuroscience; Evolutionary Analysis in Law.
Our multidisciplinary research team studies cerebral hemodynamics, cerebral blood flow and oxygen extraction fraction, in adults and children with sickle cell disease (SCD) and other medical conditions that increase stroke risk, using novel MRI measures.
Sensory-Motor Systems; Brain Plasticity; Mammalian Brain Evolution.
Impaired GABAergic Signaling in epilepsy, Autism and Brain Development.
Dr. Kavalali's group studies molecular mechanisms underlying synaptic vesicle dynamics and their impact on neuronal signaling.
Discovery of Disease Mechanisms of Comorbidities in Neuropsychiatric Disorders, Genotype-Phenotype Relations.
Affective neuroscience and vulnerability for mood and anxiety disorders; Neural predictors of response to treatment for internalizing disorders in children and adolescents.
Magnetic resonance imaging & statistical analysis with emphasis on medical imaging. His research concentrates on applying image-processing technologies to leverage large-scale imaging studies to improve understanding of individual anatomy and personalize medicine.
Social communication and interaction in autism and related neurodevelopmental disorders; music cognition; multi-method approaches including EEG, eye-tracking, acoustics, and behavior.
Using human induced pluripotent stem cells to construct biomimetic neurovascular tissues for drug screening and disease modeling applications; in vitro selection and directed evolution approaches for biosensing and therapeutic design.
The aim of our research is to increase our scientific understanding of the neural mechanisms that give rise to visual perception in order to prevent and treat visual deficits, disorders and disease.
The focus of my research is to improve the sleep, health, and well-being of individuals with autism and related conditions.
Behavioral and neural basis of human attention, Behavioral and neural basis of legal decision-making.
Better understanding neural plasticity when we adapt to new levels of stimuli, when we express daily biological rhythms, or when we learn.
We study the molecular and cellular mechanisms of somatosensory circuit assembly.
We focus on the mechanisms of protein synthesis, the regulatory role of ribosomes and the role of translation regulation in human health.
Decoding genetic programs for building and remodeling neurons.
The molecular and cellular basis of neural plasticity as it pertains to psychiatric disorders, and working to elucidate the mechanisms underlying antidepressant efficacy.
Molecular Mechanism of Synaptic Plasticity - Biochemistry and Imaging of Macromolecules in the Synapse.
The clinical care, clinical research, and translational research for genetic neurodevelopmental disorders, with an emphasis on Rett syndrome.
Pathological brain aging, particularly Alzheimer's disease, including identifying biological vulnerabilities leading to increased risk of the development of Alzheimer's disease in women and individuals with Down syndrome.
The Nguyen Lab aims to bridge our understanding of neural function and dysfunction at the level of molecules, cells, circuits, and behavior.

Understanding the pathophysiology of neurodevelopmental disorders to develop and assess novel therapeutics.
Diabetes, Endocrinology, and Metabolism.
The involvement of subcortical brain regions in sudden unexpected death in epilepsy (SUDEP), the enigmatic pathology whereby epileptic patients are found to have died without another cause.
The integration of medicinal chemistry with biomarking imaging technology for the discovery of biomarkers and molecular imaging probes, combined with drug delivery approaches dedicated to cancer and Alzheimer's disease.
Cognitive and Neural Dynamics of the Human Memory System; Neuroimaging; Electrophysiology; Computational Modeling.
How sounds are encoded in the brain by the activity of populations of neurons, and how these populations of neurons may subserve auditory perception and behavior.
The Rex laboratory uses translational approaches to study mechanisms and develop treatments for vision loss due to damage to the optic nerve from trauma or glaucoma.
The RASR laboratory develops and applies cutting-edge proteomics and other 'omics approaches to advance understanding of aging and Alzheimer's disease.
Integrative statistical models of big neuroscience networks, evolutionary principles of brain network organization, information transfer in neural systems, neuropsychiatric connectivity phenotypes.
Elucidating genetic etiology of behavioral health traits and psychiatric diagnoses, and utilizing genetics/genomics and EHR data to understand biological mechanism and interventional strategies of psychiatric disorders.
The cellular and molecular mechanisms underlying synaptic circuit assembly and function in the mammalian central nervous system.
My lab investigates hypertension as a risk factor for cognitive impairment, utilizing mouse models of hypertension to assess the mechanisms leading to cognitive decline.
The SchragLAB is focused on discovering shared molecular pathways between Alzheimer's disease and cerebral amyloid angiopathy.
Cognitive processes that contribute to symptoms of schizophrenia; psychotherapeutic interventions for psychosis; delusions; neuroimaging.
We develop animal models and leverage sophisticated technologies to elucidate the neural basis of motivation and maladaptive decision making.
Developmental Neurobiology of Limbic, Hypothalamic and Autonomic Neural Architecture.
Developmental Genetics of Neural Crest Stem Cells in Visceral Organ Innervation.
The Suver lab studies mechanisms of active sensing in Drosophila and the general principles underlying perception and behavior.
Neurobiological factors influencing the phenomenology and outcomes of late-life depression using a variety of methods including neuroimaging, ecological assessments, and neurocognitive approaches.
Scientific progress in the fields of regeneration and disease depends on a fundamental understanding of developmental processes.
fMRI, behavioral, and neurocomputational studies of visual perception, attentional selection, face and object recognition, using computational modeling and deep learning to characterize human visual processing.
The LaND lab investigates the role of action and perception in human learning and, more specifically, how the brain mediates that relationship throughout child development and in adulthood.
Multisensory Processing; Sensorimotor Transformations; Perception; Autism; Cochlear Implants; Plasticity.
Our lab focuses on uncovering the cellular and molecular mechanisms that drive neurodegeneration in the eye and, more broadly, throughout the central nervous system (CNS).
Educational neuroscience, primarily in the development of mathematical skills and neurocognitive mechanisms that enable this type of cognition.
Using electrophysiology, neurochemistry, behavioral pharmacology, reinforcement learning modeling, machine learning approaches and psychophysics to understand how cells, circuits, and networks support flexible learning and attentional control in primate brains.
The nature and neural correlates of attention and memory. His research uses a combination of behavioral, electrophysiological, imaging, and modeling methods.
The etiology and treatment of schizophrenia and related psychotic disorders using neuroimaging.
Identify brain and behavioral factors that explain heterogeneity, predict growth and differential response to treatment, and increase our understanding of how/why treatment works in young children with, or at risk for, neurodevelopmental disorders.
How epileptic networks in the whole-brain interact to trigger seizures during sleep/quiet-wakefulness and cognitive comorbidity, using physiology, optogenetic techniques and in vivo idiopathic generalized epilepsy animal models with GABAergic receptor mutations.
Computational Genomics; Bioinformatics; Algorithms for reconstructing personal and cancer genomes; Computational Neuroscience; Dynamic behavior of neural circuits; Machine learning; Artificial Intelligence.
Unraveling the in situ organization and dynamics of macromolecular assemblies at the molecular resolution, with the primary goal of gaining profound insights into their critical role in neuronal function, particularly synaptic transmission.
Molecular Genetics and Functionality of Odorant Receptors and Olfactory-Based Behavior in Malaria Vector Mosquitoes and Eusocial Ants.