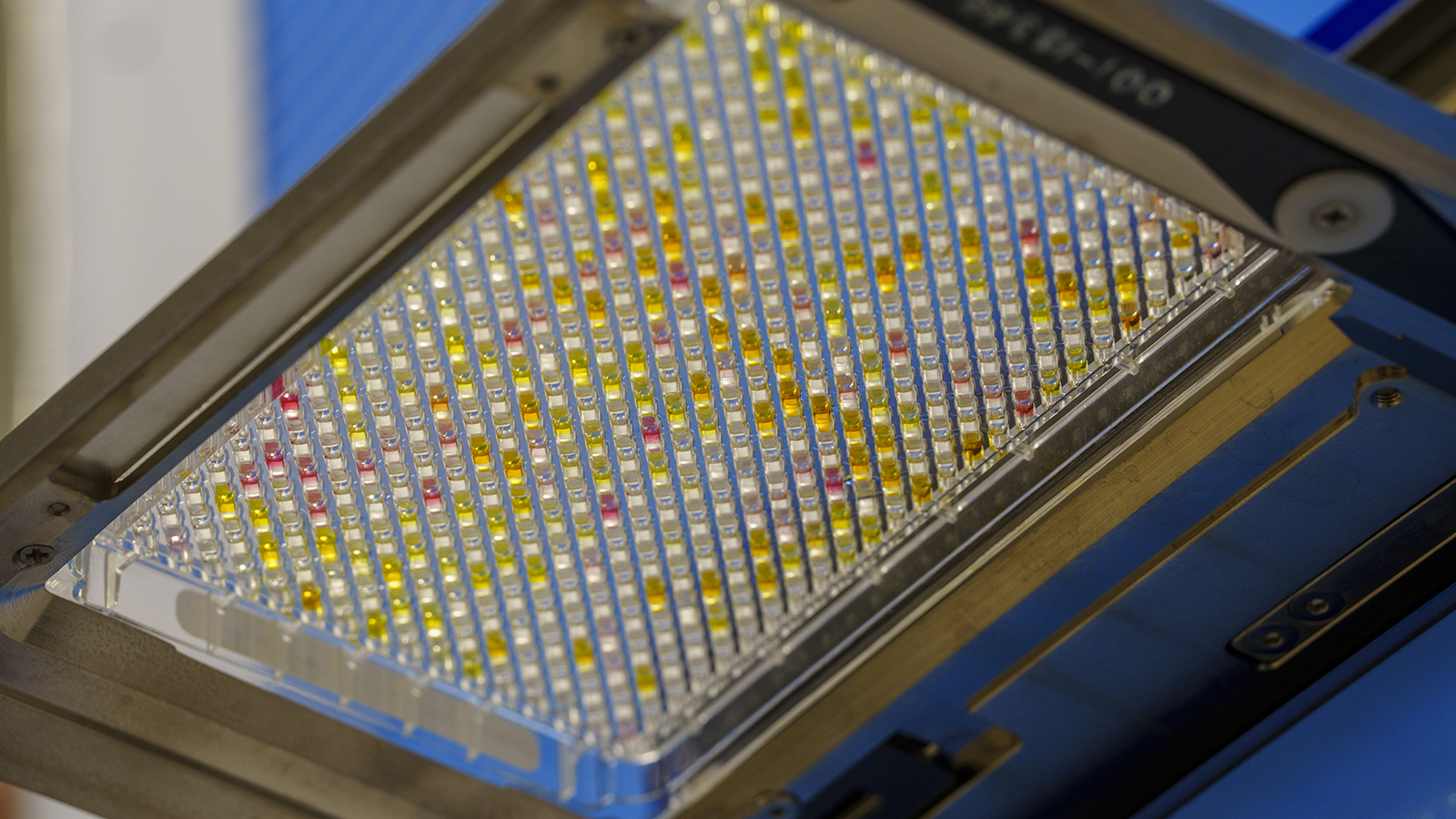
Overview
History
In 2002, Vanderbilt University made a major investment in therapeutic discovery and translation through the creation of the Vanderbilt Institute of Chemical Biology (VICB). A trans-institutional initiative between the College of Arts and Sciences and the School of Medicine, the VICB was given the mission to apply chemistry to important biological problems. Key to successful therapeutic discovery is the availability of resources enabling the high-throughput screening (HTS) of small molecules. To promote molecular probe discovery, the foundation of chemical biology research, the VICB established the V-HTS Core Facility in 2005. In the past 20 years, several landmark efforts have helped to boost the scope and capabilities of the V-HTS including the accumulation of >350,000 compounds available for screening, several high-end instruments (liquid handlers, kinetic imaging plate readers, and high-content imager), compound management automation, and robotic handling for screening automation that mimics industry-standard HTS practices. Knowing the importance of academic drug discovery, the V-HTS is further institutionally- supported by the University through the Vanderbilt Office of Research as well as the Vanderbilt Ingram Cancer Center.
Misson
The Vanderbilt Institute of Chemical Biology (VICB) High-Throughput Screening (V-HTS) Core accelerates drug discovery and biomedical breakthroughs through cutting-edge screening capabilities. Our comprehensive compound library contains over 350,000 diverse small molecules, including FDA-approved
drugs, NIH clinical candidates, and proprietary Vanderbilt synthesized compounds. The V-HTS Core offers small molecule screening, functional genomic screening with whole-genome libraries, and phenotypic screening using advanced imaging and machine learning analysis software. Our flexible, collaborative
model offers full-service, turnkey operations and walk-up access, along with personalized training. Advanced instrumentation includes multi-mode kinetic plate readers, high-content imaging systems, high-throughput metabolic instrumentation, and fully automated compound management platforms. Expert staff support researchers from assay development through hit-to-lead progression and data analysis and informatics support, ensuring reliable, reproducible results for basic research and pharmacology applications.
Our Team

Director
Assay Development and High-Throughput Screening
The assay development and HTS team is a group of expert staff responsible for developing and automating biological assays as well as conducting biochemical and cell-based high-throughput screens to identify new lead compounds that benefit both mechanistic studies and drug discovery/development. Working closely with research investigators, the HTS team strives to provide rapid, cost-effective assay development and chemical library screening. The team aids in instrument training/assistance, assay optimization, pilot screening, automation, data capture and analysis, compound distribution, full library screening, and screen validation.
Informatics
The HTS Informatics team is responsible for integrating automation and instrumentation with downstream data handling and reporting. This group maintains the laboratory technology infrastructure, including maintaining and troubleshooting instrumentation, robotic integration, network security, performing data backups, and managing data access. The team is also responsible for database management and information processing, including the Laboratory Information Management System (LIMS) management. They perform systems analysis and programming as needed for instrument interfaces and integration, as well as extracting, reformatting, uploading, aggregating, and/or analyzing data gathered in the course of HTS experiments. In addition, the group assists laboratory and scientific personnel with all their informatics needs, including overseeing data exchanges with research investigators.
Compound Management
The HTS facility houses a state-of-the-art automated compound management system (Hamilton Storage Technologies) that provides environmentally controlled storage and on-demand access to the library compounds. The chemical library collection consists of commercially purchased libraries as well as compounds synthesized by collaborating investigators to give a diverse array of chemical materials for biological testing. Processes such as compound registration, storage, and handling all meet or exceed industry standards. Compounds can be dispensed into a variety of tube and plate formats (6-well to 384-well) to accommodate the research investigator’s needs.
Lab Management
The HTS facility requires a dedicated person to maintain and manage inventory, supplies, and instruments.
Internal Advisory Board
The Scientific Advisory Board for the Vanderbilt HTS Facility is an association of research investigators and leaders with proven track records in academic and industrial research programs. Together, they oversee the intermediate and long-term goals of the facility by advising on growth and diversification strategies, assessing and prioritizing proposed projects that enter the facility, critiquing success metrics, and providing financial oversight and funding leadership.
How to get started
HTS Policies
Acknowledge
Publication Guidelines:
As you near publishing work that used HTS resources, please consider the following:
Small Molecule Naming
When referring to Vanderbilt library small molecules and other registered compounds in presentations, we encourage you to use the full 7-digit VU#, e.g. VU0824980. This provides a unique reference and allows proper tracking.
Authorship
Some of the projects that we support require significant intellectual input from HTS research faculty and staff in a way to warrant co-authorship on publications and listing as co-PI on grants. Examples include contributing to the design and development of an assay and determining the approach for and carrying out data analysis.
We request that the recommendations for authorship of the International Committee of Medical Journal Editors (ICMJE) be followed and that the PI engages HTS collaborators during the project and manuscript preparation. According to the ICMJE recommendations, individuals who meet all of the following criteria should be included as co-authors:
- Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; AND
· Drafting the work or revising it critically for important intellectual content; AND
· Final approval of the version to be published; AND
· Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Depending on the resource(s) that you use within HTS (including Compound Management and Informatics), a general or more specific acknowledgment may be appropriate. Below are examples of acknowledgements to include.
* NIH requires citation of S10 grant for instruments purchased using these funds. The Seahorse falls in this category.
Resource | Example Acknowledgement |
HTS Instruments | Experiments were performed in the Vanderbilt High-Throughput Screening (HTS) Core Facility with assistance provided by <staff member names>. The HTS Core receives support from the Vanderbilt Institute of Chemical Biology and the Vanderbilt Ingram Cancer Center (P30 CA68485). |
Seahorse Analyzer* | The Agilent Seahorse Extracellular Flux Analyzer is housed and managed within the Vanderbilt High-Throughput Screening Core Facility, an institutionally supported core, and was funded by NIH Shared Instrumentation Grant 1S10OD018015. |
Panoptic 2* | The WaveFront Biosciences Panoptic kinetic imaging plate reader is housed and managed within the Vanderbilt High-Throughput Screening Core Facility, an institutionally supported core, and was funded by NIH Shared Instrumentation Grant 1S10OD021734. |
NIH Clinical Collections | The NIH Clinical Collection is provided through the National Institutes of Health Molecular Libraries Roadmap Initiative and was distributed by the Vanderbilt High-throughput Screening Core Facility. The HTS Core receives support from the Vanderbilt Institute of Chemical Biology and the Vanderbilt Ingram Cancer Center (P30 CA68485). |
SelleckChem FDA Approved | The FDA approved library was provided by the Vanderbilt CTSA (UL1TR00044) and distributed by the Vanderbilt High-Throughput Screening Core Facility. The HTS Core receives support from the Vanderbilt Institute of Chemical Biology and the Vanderbilt Ingram Cancer Center (P30 CA68485). |
SelleckChem Anti-cancer and Protein Kinase Inhibitor Collections | The anti-cancer and protein kinase inhibitor collections were provided by funds donated by the Vanderbilt Ingram Cancer Center (P30 CA68485), the Vanderbilt Center for Cancer Targeted Therapies (part of the VICC), Dr. Lawrence J. Marnett, and Dr. Michael Savona. |
Marnett Library | The Marnett library was donated by Lawrence J. Marnett. |
Other Libraries | The <collection name> was distributed by the Vanderbilt High-Throughput Screening Core Facility. The HTS Core receives support from the Vanderbilt Institute of Chemical Biology and the Vanderbilt Ingram Cancer Center (P30 CA68485). |