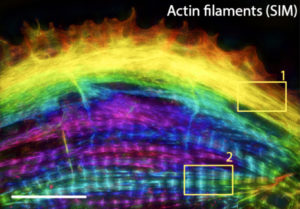
The sarcomere is a repeating unit of interdigitating actin and myosin filaments that serves as the building blocks of the myofibrils in striated muscle cells. Sarcomeres are demarcated at each end by a Z-disc containing α-actinin 2 to which thin fibers of actin are attached. In the center are the A-bands comprising thick filaments composed of myosin. ATP-driven movement of myosin along the actin filaments brings the Z-discs closer together, thereby leading to muscle contraction. Although we have learned much about their structure and function, the mechanism by which sarcomeres are assembled, particularly in cardiomyocytes, is not well understood. Thus, Vanderbilt Basic Sciences investigators Dylan Burnette and Matt Tyska, along with their laboratories and collaborators, explored the process in cultured human induced pluripotent stem cell-derived cardiomyocytes (hiCMs). Prior work had shown that near the edge of cardiomyocytes is a network of actin filaments that resemble actin arc stress fibers in other cells. The cardiomyocyte sarcomeres are located more centrally in the cell. As the stress fibers in cardiomyocytes contain non-muscle myosin IIB (NMIIB) along with muscle-associated proteins, some researchers have hypothesized that they serve as a template for sarcomere formation. Others, however, have suggested that the sarcomere is “stitched together” from separate pools of preassembled parts. The Burnette lab investigators began to address this controversy through careful observation of sarcomere formation in hiCMs. They found that soon after plating, hiCMs acquired actin-containing muscle stress fibers (MSFs) along their edge, with sarcomeres appearing several hours later. α-Actinin was visible as puncta in association with both MSFs and sarcomeres, and the researchers verified that it became incorporated into the sarcomere Z-discs. As previously reported for actin stress fibers in other cells, the actin in MSFs exhibited retrograde flow away from the edge of the cell, but this flow was much slower than that seen in non-muscle cells. Furthermore, whereas the cytoskeleton-regulating complex Arp2/3 is required for actin stress fiber assembly in non-muscle cells, an inhibitor of this complex had no effect on MSF or sarcomere formation in hiCMs. These observations demonstrated that MSFs are not structurally or functionally identical to stress fibers of non-muscle cells. In contrast, as in the case of non-muscle stress fibers, formins were needed to support MSF formation, and siRNA-mediated gene expression knockdown demonstrated that the formin FHOD3 played a particularly important role. Both NMIIA and NMIIB are required for non-muscle stress fiber formation, and both non-muscle myosins were present in MSFs. The researchers used siRNA to show that depletion of NMIIA or NMIIB partially or completely blocked sarcomere formation in hiCMs, despite the fact that neither protein is a sarcomere component. The primary myosin in hiCM sarcomeres is βCMII. As expected, most of this protein in hiCMs was localized to sarcomeres; however, a fraction was also found in a region in the cell that contained NMIIB, and co-filaments of the two proteins were present in this region. The researchers went on to show that disruption of actin filaments led to failure of sarcomere formation and that the A-bands of myosin filaments in sarcomeres formed by concatenation of individual filaments as opposed to expansion of existing tight bundles of filaments. Together, the results suggested that both prior hypotheses regarding sarcomere assembly were partially correct. It appears that, although MSFs are not identical to non-muscle cell stress fibers, they do serve as a template or precursor for sarcomere formation. Both NMIIA and NMIIB are required for sarcomere assembly, and intact MSFs must also be present. As MSFs move from the edge of the cell toward the center, they gradually change composition, with non-muscle myosins being replaced with βCMII. Notably, the existence of co-filaments of non-muscle and cardiac muscle myosins had not been reported prior to these studies. At the same time, the researchers also found support for the “stitching” hypothesis in the case of myosin fiber assembly. These findings provide critical new insight into cardiomyocyte sarcomere formation. The work is published in the journaleLife [A. M. Fenix, et al. (2018) eLife, published December 12, DOI: 10.7554/eLife.42144].