This article was submitted by the senior author of the featured paper, Lauren P. Jackson.
Human cells contain a “FedEx system” to ensure that important protein and fatty lipid cargo molecules are delivered to the right destination at the right time. Large protein coat complexes build structures that sort protein and lipid molecules into small round (vesicular) or elongated, tube-shaped membrane structures. These vesicles or tubules then shuttle cargo molecules to the correct cellular destination. Delivery failures are linked to many human diseases, including neurological disorders like Alzheimer’s and Parkinson’s diseases.
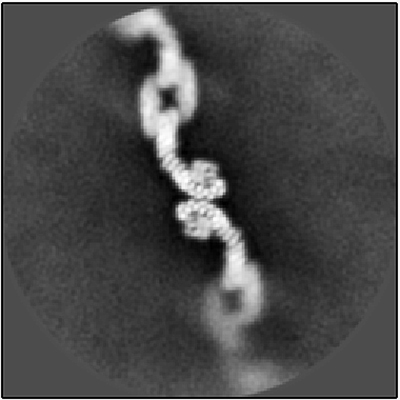
My lab, part of the Departments of Biological Sciences and Biochemistry, used a cutting-edge structural biology method called single-particle cryo-electron microscopy (cryo-EM) to uncover new structures of a highly conserved protein complex called retromer. Retromer is located on a busy sorting station called the endosome, where it directs important membrane-spanning proteins to different destinations. It ferries protein cargoes such as glutamate receptors, which are important in neurotransmission, and SorLA, a protein that binds and regulates amyloid precursor protein (APP), precursor to the amyloid beta plaques that are found in the brains of people with Alzheimer’s. A major goal in the field tries to address how a single protein complex sorts multiple cargoes to different destinations.
Our recent research, published in Structure, shows that retromer can assemble into multiple architectures or shapes by determining the structures of these different forms. Cryo-EM allowed us to directly visualize tens of thousands of protein molecules. We first sorted the images of retromer assemblies by their size and shape, which allowed us to then obtain structures of each architecture, and then used these structural models to test amino acid residues in various interfaces. Through this process, we identified key amino acids that hold the protein assemblies together.
This work indicates that retromer is a flexible scaffold, which could allow this complex to sort different cargoes. A key mutation in one subunit (an aspartic acid-to-asparagine mutation in a protein called VPS35) sits near a key assembly interface. Our work with these structures suggests that this mutation may cause an assembly defect over time that is linked to late-onset Parkinson’s. Our latest research provides the foundation for determining whether retromer could be a therapeutic target: small molecules could potentially stabilize retromer assemblies and promote movement of important protein cargoes that are critical for neurological function.
This work was supported by the National Institutes of Health and the Pew Charitable Trusts.