When we think of the age-old adage about getting old, “What new ache or pain will each new day bring?” we often imagine ailments such as joint or bone pain, a hyperactive bladder, or even memory loss, but Kevin Schey, Stevenson Professor of Biochemistry at the School of Medicine Basic Sciences, thinks a lot about the loss of eyesight.

According to the National Eye Institute, most older adults who experience vision loss can blame it on age-related macular degeneration, and approximately 11 million people in the U.S. have the disease. Although AMD doesn’t cause total blindness, it can significantly affect quality of life by blurring a person’s central vision, making it difficult to carry out day-to-day tasks such as reading, driving, or even seeing other people’s faces.
One contributing factor in diagnosing AMD is the presence of “hyperreflective foci” in the retina—the light-sensing part of the eye—using a technique called optical coherence tomography. The foci are small, discrete lesions in the retina that have high reflectivity and can be reliably tracked with OCT, serving as biomarkers for the disease.
Schey and colleagues set out to dig into the molecular and cellular nature of hyperreflective foci in and found a role for lipids in age-related vision loss, paving the way for potential new therapies. Their work was published in PNAS in July.
We sat down with Schey, one of the study’s two leading authors (with Christine Curcio from the University of Alabama at Birmingham), who told us more about the research.
What issue/problem does your research address?
We set out to dig into the molecular and cellular nature of hyperreflective foci in OCT scans of retinas with age-related macular degeneration. We believe that retinal pigment epithelium or RPE cells can undergo changes and migrate from their normal positions in the RPE monolayer and become the source of clinically visible hyperreflective foci. There are other potential sources of hyperreflectance in the retina, but our work aimed to assess the role of RPE cells as a key source of this biomarker signal.
What was unique about your approach to the research?
We used near single-cell resolution imaging mass spectrometry, or IMS, to determine the molecular constituents of migrating RPE cells in human donor retinas. IMS was pioneered at Vanderbilt at the cutting-edge facilities within the Mass Spectrometry Research Center and it facilitated our analysis.
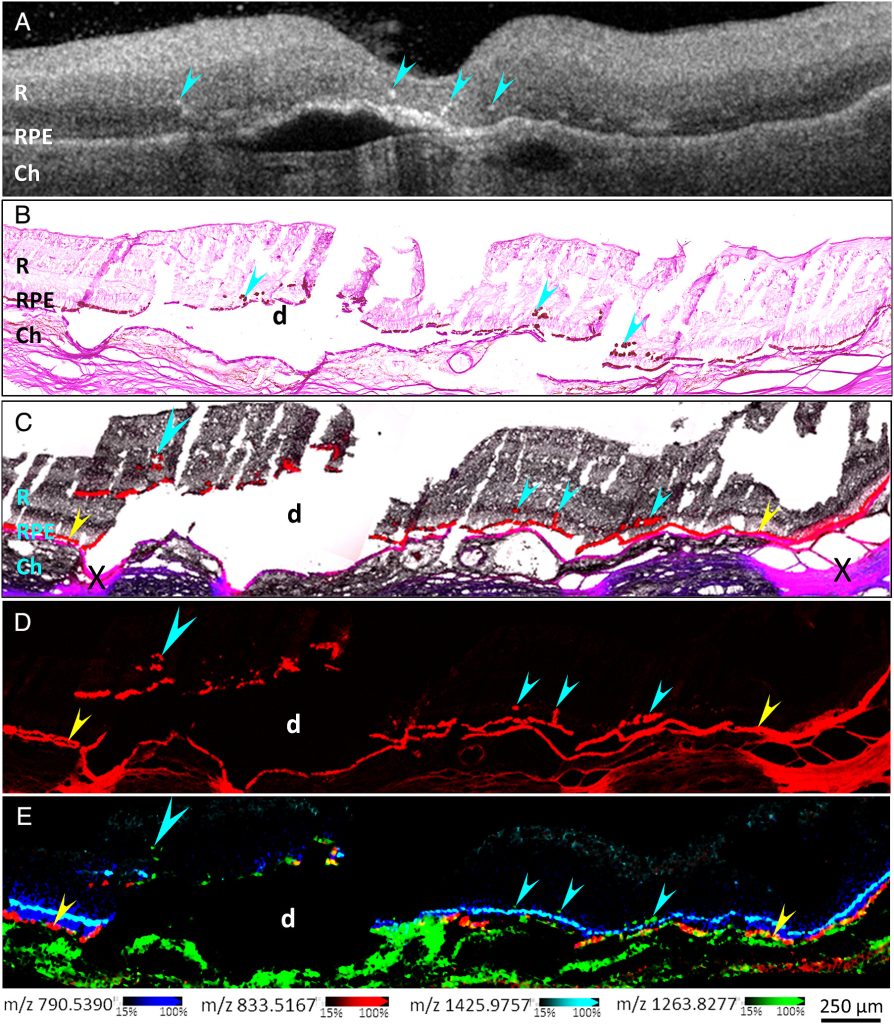
What were your top three findings?
We found that the molecular signatures of hyperreflective cells in AMD eyes are consistent with ectopic (migrating) RPE cells. As RPE cells migrate into the inner retina, changes in their molecular composition were consistent with transdifferentiation, a process that morphs the normally static epithelial cells into mesenchymal cells, which are characterized by migratory and invasive properties. We also found that the ectopic RPE cells both gain some functions they didn’t have in the RPE layer and lose some functions they had before, which we believe is caused by altered lipid metabolism in the ectopic cells. We believe that this change in lipid metabolism drives migration and the creation of the hyperreflective foci and leads to the progression of AMD.
What do you hope will be achieved with the research results on the short term?
Right now, we believe that this research confirms that some hyperreflective foci observed in OCT scans occur due to transdifferentiating RPE cells and that lipid metabolism is altered in these cells.
What are your highest translational/clinical aspirations that might come from this research?
New therapies for slowing or preventing AMD could come of our findings if we or other researchers could find ways to delay or prevent RPE transdifferentiation.
Who or what made the difference in your research?
Having access to human donor eyes with very short post-mortem times was critical for our work and was facilitated by a close collaboration with the Advancing Sight Network of Alabama. Additionally, a close partnership with Dr. Christine Curcio’s lab at the University of Alabama at Birmingham was instrumental for sample preparation, histological analysis, and data interpretation. Finally, access to state-of-the-art imaging mass spectrometry technologies within the Vanderbilt MSRC was the gel that brought everything together.
Where is this research taking you next?
We will continue to examine AMD eyes with hyperreflective foci to identify other cell types, such as microglial cells, that may be involved in the disease. We will also be focusing on the cellular environment that can lead to RPE migration and transdifferentiation.
Go deeper
The paper “Glycolipids implicated as mediators of clinically visible retinal pigment epithelial migration in age-related macular degeneration” was published in PNAS in July 2025. Zhen Wang and David Anderson are co-first authors.
The study was published open access through a transformative agreement negotiated by Vanderbilt University’s Jean and Alexander Heard Libraries. Transformative agreements eliminate traditional paywalls and remove the obstacle of article processing charges, ensuring immediate and unrestricted access to research worldwide. Vanderbilt authors can learn more about the Heard Libraries’ agreements supporting open access publishing in this research guide.
Funding
This research used funds from the National Eye Institute, Research to Prevent Blindness, and the EyeSight Foundation of Alabama.
School of Medicine Basic Sciences shared resources
This research made use of the Tissue Imaging Core and the Mass Spectrometry Core, both part of the Mass Spectrometry Research Center.