Gαo represses insulin secretion by reducing vesicular docking in pancreatic beta-cells
Gαo represses insulin secretion by reducing vesicular docking in pancreatic beta-cells.
Zhao A, Ohara-Imaizumi M, Brissova M, Benninger RK, Xu Y, Hao Y, Abramowitz J, Boulay G, Powers AC, Piston D, Jiang M, Nagamatsu S, Birnbaumer L, Gu G.
Department of Cell and Developmental Biology, Vanderbilt University Medical Center, Nashville,Tennessee, USA.
Abstract
OBJECTIVE: Pertussis toxin uncoupling-based studies have shown that Gαi and Gαo can inhibit insulin secretion in pancreatic β-cells. Yet it is unclear whether Gαi and Gαo operate through identical mechanisms and how these G-protein-mediated signals inhibit insulin secretion in vivo. Our objective is to examine whether/how Gαo regulates islet development and insulin secretion in β-cells.
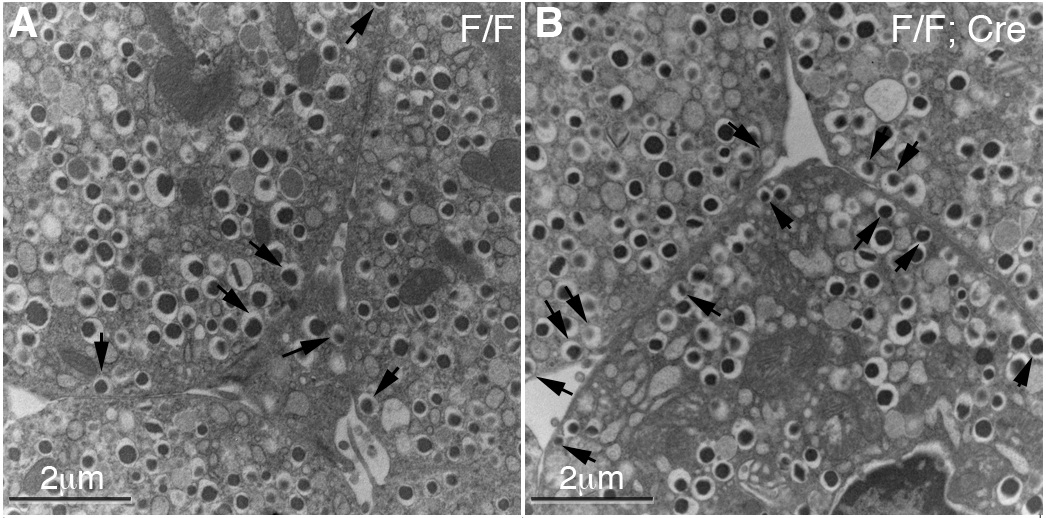
RESEARCH DESIGN AND METHODS: Immunoassays were used to analyze the Gαo expression in mouse pancreatic cells. Gαo was specifically inactivated in pancreatic progenitor cells by pancreatic cell-specific gene deletion. Hormone expression and insulin secretion in response to different stimuli were assayed in vivo and in vitro. Electron microscope and total internal reflection fluorescence-based assays were used to evaluate how Gαo regulates insulin vesicle docking and secretion in response to glucose stimulation.
RESULTS: Islet cells differentiate properly in Gαo(-/-) mutant mice. Gαo inactivation significantly enhances insulin secretion both in vivo and in isolation. Gαo nullizygous β-cells contain an increased number of insulin granules docked on the cell plasma membrane, although the total number of vesicles per β-cell remains unchanged.
CONCLUSIONS: Gαo is not required for endocrine islet cell differentiation, but it regulates the number of insulin vesicles docked on the β-cell membrane.
PMID: 20622165 [PubMed – indexed for MEDLINE]