The Purkinje neuron is a central regulator of cerebellar neurogenesis
The Purkinje Neuron Acts as a Central Regulator of Spatially and Functionally Distinct Cerebellar Precursors
Jonathan T. Fleming, Wenjuan He, Chuanming Hao, Tatiana Ketova, Fong C. Pan, Christopher C.V. Wright, Ying Litingtung, Chin Chiangemail
DOI: http://dx.doi.org/10.1016/j.devcel.2013.10.008
Highlights
•Purkinje neurons signal bidirectionally to expand functionally divergent cell types
•Nascent white matter harbors molecularly distinct but lineally related astroglia
•Shh signaling maintains the neurogenic niche in nascent white matter
•Ptf1a expression marks transiently amplifying GABAergic progenitor pools
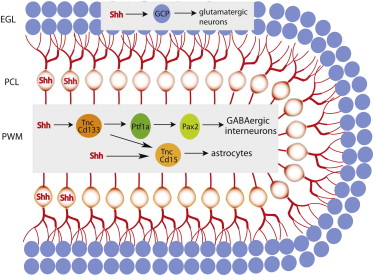 Summary
Summary
The prospective white matter (PWM) in the nascent cerebellum contains a transient germinal compartment that produces all postnatally born GABAergic inhibitory interneurons and astrocytes. However, little is known about the molecular identity and developmental potential of resident progenitors or key regulatory niche signals. Here, we show that neural stem-cell-like primary progenitors (TncYFP-low CD133+) generate intermediate astrocyte (TncYFP-low CD15+) precursors and GABAergic transient amplifying (Ptf1a+) cells. Interestingly, these lineally related but functionally divergent progenitors commonly respond to Sonic hedgehog (Shh), and blockade of reception in TNCYFP-low cells attenuates proliferation in the PWM, reducing both intermediate progenitor classes. Furthermore, we show that Shh produced from distant Purkinje neurons maintains the PWM niche independently of its classical role in regulating granule cell precursor proliferation. Our results indicate that Purkinje neurons maintain a bidirectional signaling axis, driving the production of spatially and functionally opposed inhibitory and excitatory interneurons important for motor learning and cognition.