Coupling chromosome segregation and cytokinesis involves signaling scaffold ubiquitination
EMBO J. 2011 Jan 19;30(2):341-54. Epub 2010 Dec 3.
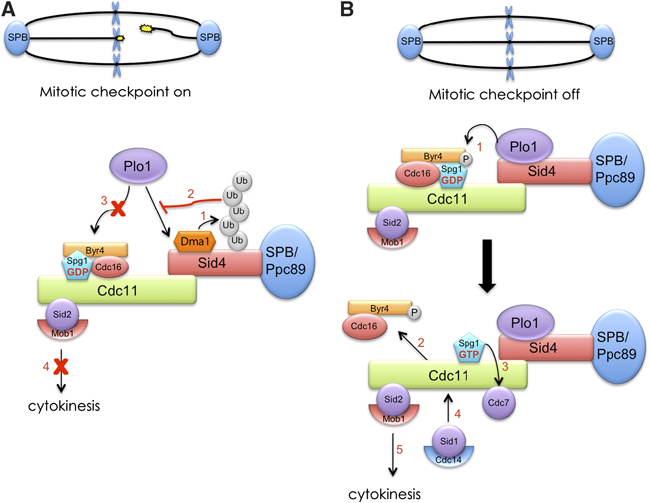
Dma1 ubiquitinates the SIN scaffold, Sid4, to impede the mitotic localization of Plo1 kinase.
Johnson AE, Gould KL.
Department of Cell and Developmental Biology, Vanderbilt University School of Medicine, Nashville, TN, USA.
Abstract
Proper cell division requires strict coordination between mitotic exit and cytokinesis. In the event of a mitotic error, cytokinesis must be inhibited to ensure equal partitioning of genetic material. In the fission yeast, Schizosaccharomyces pombe, the checkpoint protein and E3 ubiquitin ligase, Dma1, delays cytokinesis by inhibiting the septation initiation network (SIN) when chromosomes are not attached to the mitotic spindle. To elucidate the mechanism by which Dma1 inhibits the SIN, we screened all SIN components as potential Dma1 substrates and found that the SIN scaffold protein, Sid4, is ubiquitinated in vivo in a Dma1-dependent manner. To investigate the role of Sid4 ubiquitination in checkpoint function, a ubiquitination deficient sid4 allele was generated and our data indicate that Sid4 ubiquitination by Dma1 is required to prevent cytokinesis during a mitotic checkpoint arrest. Furthermore, Sid4 ubiquitination delays recruitment of the Polo-like kinase and SIN activator, Plo1, to spindle pole bodies (SPBs), while at the same time prolonging residence of the SIN inhibitor, Byr4, providing a mechanistic link between Dma1 activity and cytokinesis inhibition.