Mechanism of brush border assembly discovered
Intestinal Brush Border Assembly Driven by Protocadherin-Based Intermicrovillar Adhesion
Cell – Volume 157, Issue 2, 10 April 2014, Pages 433–446
Scott W. Crawley, David A. Shifrin Jr., Nathan E. Grega-Larson, Russell E. McConnell, Andrew E. Benesh, Suli Mao, Yuxi Zheng, Qing Yin Zheng, Ki Taek Nam, Bryan A. Millis, Bechara Kachar, Matthew J. Tyskaemail
Highlights
•Packing of brush border microvilli is driven by adhesion between protrusion tips
•Intermicrovillar adhesion complexes are composed of PCDH24 and MLPCDH
•PCDH24 and MLPCDH target to microvillar tips via interactions with harmonin and Myo7b
•Mice lacking the Usher syndrome gene harmonin exhibit defects in brush border assembly
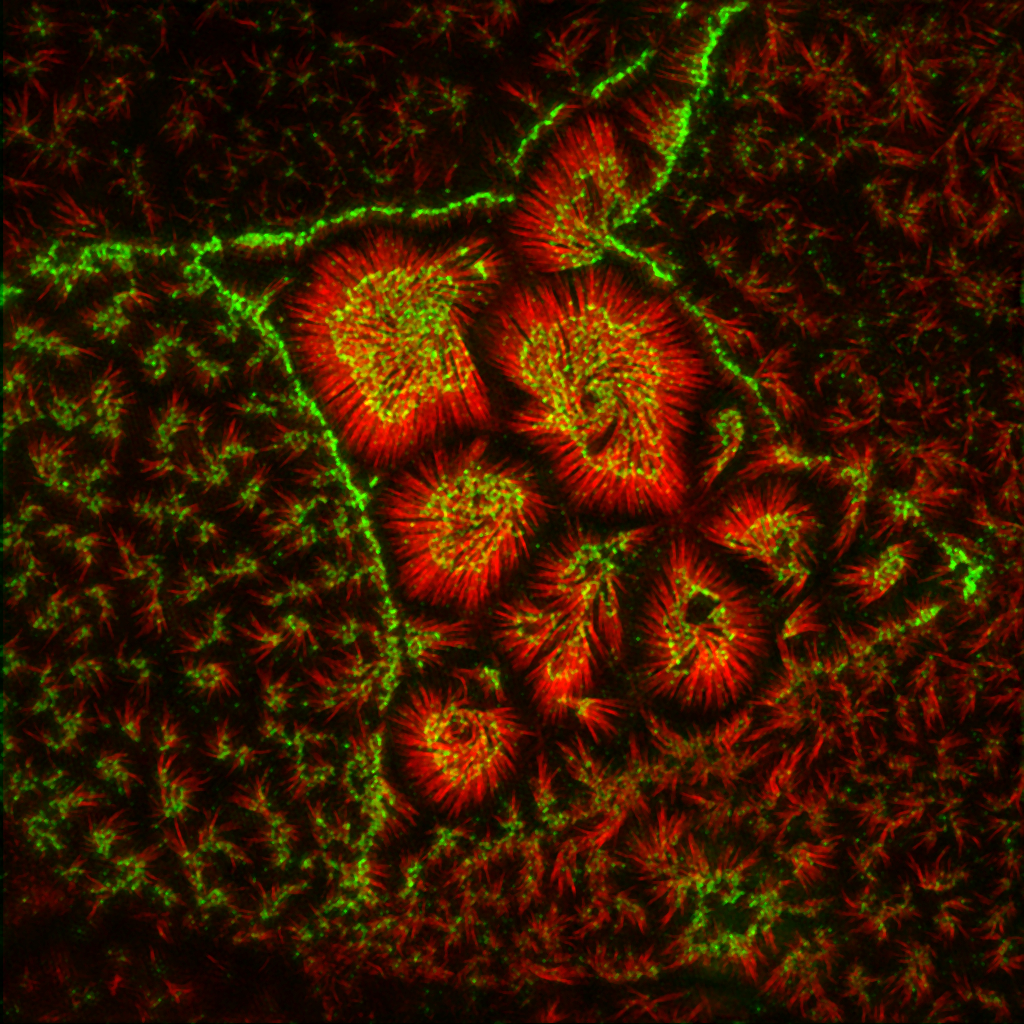
Summary
Transporting epithelial cells build apical microvilli to increase membrane surface area and enhance absorptive capacity. The intestinal brush border provides an elaborate example with tightly packed microvilli that function in nutrient absorption and host defense. Although the brush border is essential for physiological homeostasis, its assembly is poorly understood. We found that brush border assembly is driven by the formation of Ca2+-dependent adhesion links between adjacent microvilli. Intermicrovillar links are composed of protocadherin-24 and mucin-like protocadherin, which target to microvillar tips and interact to form a trans-heterophilic complex. The cytoplasmic domains of microvillar protocadherins interact with the scaffolding protein, harmonin, and myosin-7b, which promote localization to microvillar tips. Finally, a mouse model of Usher syndrome lacking harmonin exhibits microvillar protocadherin mislocalization and severe defects in brush border morphology. These data reveal an adhesion-based mechanism for brush border assembly and illuminate the basis of intestinal pathology in patients with Usher syndrome.