Are you sitting on a game-changing idea that could revolutionize your industry? Don't let it gather dust—Vanderbilt University’s expert tech transfer team is here to help you bring it to life. With a proven track record of transforming Vanderbilt researchers’ work into successful business ventures, they possess the knowledge, resources, and connections to transform your innovation from concept to reality.
Vanderbilt’s Center for Technology Transfer and Commercialization group focuses on commercializing new technologies, drugs, and therapeutics developed through university research. They work to protect intellectual property, identify potential commercial partners, and facilitate technology licensing agreements. Their goal is to transform innovative discoveries into real-world applications that benefit society.
The CTTC came into existence in the wake of the 1980 Bayh-Dole Act, also known as the Patent and Trademark Law Amendments Act. The legislation allowed universities, research institutions, and other nonprofit organizations to own, protect, and commercialize federally funded inventions.

“That created the tech transfer industry,” said CTTC Assistant Vice Chancellor Alan Bentley. The mission of the CTTC, which was founded 11 years later, is to “assist inventors in bringing their innovations to practical application for the benefit of the public, and then to help ensure investigators’ research achieves impact for the greater good,” Bentley also said.
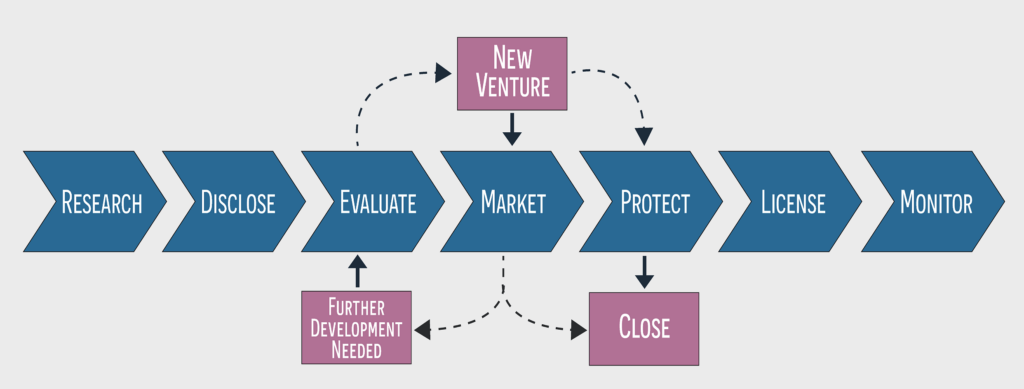
Since its inception, the CTTC has founded 118 new companies and averages about eight new startups and 200 invention disclosures per year. Its main services, available to VU and Vanderbilt University Medical Center faculty and staff, are licensing inventions, forming startups, and securing industry funding, which often takes the form of a collaboration between academia and a private company. Graduate students and postdocs are typically listed on an invention disclosure or a patent under their advisor.
Yet many Vanderbilt researchers either don’t know they have a patentable idea or are intimidated by the process of taking an idea to market. The CTTC’s job is to help identify commercially viable ideas and smooth their path to market.
The track record
One notable tech transfer success involves a therapeutic treatment for Alzheimer's disease developed by Craig Lindsley and Jeff Conn, co-founders of the Warren Center for Neuroscience Drug Discovery. Vanderbilt entered into an exclusive worldwide licensing and collaboration agreement with San Diego-based Acadia Pharmaceuticals, which develops and commercializes treatments for central nervous system disorders, such as Alzheimer’s disease and schizophrenia.
The collaboration focuses on positive allosteric modulators of the muscarinic acetylcholine receptor M1, G protein-coupled receptors involved in the parasympathetic nervous system. A lead compound—VU319, now in phase 1 clinical trials—was discovered by WCNDD researchers and could help slow memory loss accompanying serious brain disorders. In addition to VU319, the licensing agreement with Acadia includes other compounds in preclinical development, as well as those in an ongoing discovery program based on early-stage research into fundamental biological mechanisms.

“The tech transfer team was extremely easy to work with,” said Tina Iverson, Louise B. McGavock Professor of Pharmacology. “We almost had to do nothing.” To help Iverson commercialize [her team’s invention], the CTTC filed a provisional patent based on interviews with her team, a grant, and an unpublished manuscript. As her patent involved researchers from other institutions, the CTTC worked with their offices to process the patent. After a year, they converted the provisional patent to a full patent. “The CTTC advised us at early stages about our likelihood of success and advised us about what we needed to do to protect the patent space before we filed,” Iverson said. "I would encourage people to contact CTTC with their idea earlier, rather than later, to help guide the process.”
Perhaps the CTTC’s greatest success story to-date has been a monoclonal antibody combination that protects against COVID-19 discovered by James Crowe, director of the Vanderbilt Vaccine Center at VUMC. The commercialized product, Evusheld®, was optimized and developed by AstraZeneca and received emergency authorization from the U.S. Food and Drug Administration in December 2021. It is the first approved preventative treatment for adults and children 12 years and older who have compromised immune systems before exposure to the virus. “That has been our biggest success in terms of direct impact on patient lives, and it is our largest royalty generator for the university,” Bentley said.

Taking the first steps
What is the first step a Vanderbilt researcher should take if they have an idea for an invention? Fill out the CTTC’s new Concept Submission Form. “Fill this form out if you even have a hint of an idea that you think may have commercial potential,” Bentley said. “Just submit a few sentences and our staff will go through it and see if there's any potential viability for that idea.”
Got an idea to run by the tech transfer team for a rapid evaluation for commercial potential? Go to the CTTC website, and click on the large “Contact Submission Form” button!
Once an idea has been submitted, the CTTC wheels start turning. Depending on the type of idea, their biotech licensing team or their engineering team evaluate it in conjunction with the inventors to determine if it has any legs. “Is the idea novel? Can it be readily productized? What competing solutions exist? Are we able to secure intellectual property protection?” Bentley said. “We have a rigorous protocol for evaluating the protectability and marketability of a new idea, and we progress through it transparently with the researchers.”

“Our office has negotiated more pharmaceutical licensing and co-development agreements with large pharmaceutical and biotechnology companies than most any other university in the country,” said Mike Villalobos, assistant director for life science licensing. “We have experience with complex deal structures, have worked through every kind of contract issue imaginable, and generally secure value well beyond what most universities see for these types of agreements.”
Innovation ambassadors
The Innovation Ambassador Program, launched in 2022 by the CTTC in collaboration with VUMC and The Wond’ry, Vanderbilt’s center for innovation, provides a resource for faculty to learn more about inventions and technology commercialization directly from their peers. Innovation ambassadors receive about six hours of initial training in commercialization, intellectual property, entrepreneurship, relevant university policies and procedures, and more. They’re also kept abreast of new programs and processes that get rolled out across campus. The goal is to enable the ambassadors to share the information and resources that can help other researchers in their departments protect and commercialize their ideas and inventions.
“The main goal of an innovation ambassador is to be part of an overall ecosystem that encourages and facilitates entrepreneurship and commercialization of discoveries made within the Vanderbilt and VUMC communities,” said Rob Carnahan, professor of pediatrics and an inaugural member of the ambassador program. “Trained ambassadors will more likely spot ideas with commercialization potential through their everyday work and interactions. They can then encourage and guide their peers to explore these possibilities.” Developing and transferring discoveries into practical usage helps maximize the impact of Vanderbilt research on the world.
One ambassador, Alex Waterson, research professor of pharmacology, is also a tech transfer client. “The CTTC has been a great resource for our research group over the last several years. They not only have helped us file patent applications on our inventions, but they have managed most of the post-filing patent review process,” Waterson said. “They are also a big part of our interactions with both academic collaborators and corporate partners, assisting with everything from simple material transfer agreements to complex, multi-institutional licensing agreements. Their expertise in these types of things allows us to concentrate on pushing the science forward.”
Contact the CTTC—Before you publish!

Some researchers with good ideas for new products don’t act on them. As CTTC General Manager of Corporate Alliances Margaret Read explained, it often has to do with article publication. “Sometimes people think that if they file a disclosure, it means they'll be prohibited from publishing on it. The truth is very different,” Read said. “If you are about to publish or present research and you think there is a remote possibility that there can be intellectual property in that work, please reach out and let us know. In most cases, our office can act to protect such disclosures on the same day.” In cases where there is IP involved, not contacting the CTTC can destroy its value, particularly outside of the U.S., where there is no grace period to protect ideas once they are made public. “Last-minute disclosures are not ideal, but doing it at the last minute is better than not doing it at all,” Read said.
The CTTC is well positioned to help researchers preserve and create value around their ideas from the beginning of the process through the end. According to Read, there can be potential road blocks that can pop up, from obtaining grant funding and IP protection to finding good pharmaceutical or industry partners to take drugs through Investigational New Drug-enabling studies and clinical trials.
Pushing the gender barrier
CTTC reached another significant milestone this year: More female inventors were named on disclosures than male inventors during fiscal year 2024. “That’s not a coincidence,” Read said. “The CTTC has been working hard to promote women inventors at Vanderbilt, and they are becoming more prominent within the community." CTTC is working diligently to match female innovators with people who can help them. Recently, CTTC sponsored three female inventors at a Women-in-Science conference in New York City hosted by Deerfield Management.
“We are seeing more female researchers pursuing startup opportunities as well,” Bentley said. “Of the last dozen start-ups we have launched or about to launch, more than half are led by female faculty entrepreneurs.”
As complex—and at times frustrating—as shepherding a new idea to market can be, it’s the challenge that motivates Bentley to come to work each day. “I got into the business because I was told that being a tech transfer professional offered me the opportunity to learn a little bit about a whole bunch of different cutting-edge fields,” he said. “Being on the forefront of what's happening is invigorating, and every five or so years you get to see a product that you helped move through the system actually have a major impact on people’s lives.”
By bridging the gap between academic research and industry, the CTTC continues to play a crucial role in bringing Vanderbilt's biomedical innovations to the market. If you’re ready to stop dreaming and start disrupting, it’s time to partner with Vanderbilt and unleash your ideas’ true potential.
