Cryopreservation and Rederivation
Cryopreservation
Cryopreservation provides a safe path for preserving the quality and protection of the genome edited mouse line for any potential projected future experiments.
Cryopreservation benefits include:
- Safeguards valuable mouse strains against catastrophe
- Limits genetic drift
- Protects lines from disease outbreak
- Protects lines from breeding cessation
- Eases distribution of mouse lines without the need of shipping live animals
- Reduces housing and genotyping costs
Assisted Reproduction Technologies
VGER (Vanderbilt Genome Editing Resource) offers a range of services aimed at introducing new transgenic lines or removing pathogens from mouse facilities. These services encompass in vitro fertilization, rederivation, and ovarian transplantation, resulting in the generation of Specific Pathogen-Free animals. While the importation of cryopreserved sperm or embryos is permitted, it requires prior approval from the Vanderbilt Department of Animal Care. To initiate the process, the principal investigator (PI) must first submit an animal order in TOPAZ and subsequently submit an iLab service request upon receiving approval.
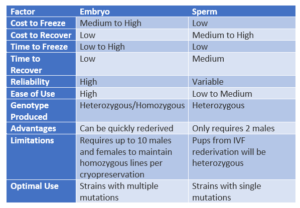
Rederivation of cryopreserved sperm or embryos is contingent upon several factors, including genetic background, sample history, and employed protocols. VGER provides three options for rederiving mouse lines from external sources: in vitro fertilization, frozen embryo cryorecovery, and fresh embryo rederivation. For mouse lines maintained at Vanderbilt, VGER offers in vitro fertilization and line expansion via IVF. Homozygous lines or those with multiple genetic mutations are recommended to undergo rederivation through embryo transfer or embryo retrieval and transfer.
Prior to arranging shipment to VGER, the importation of cryopreserved sperm or embryos necessitates pre-approval from the Vanderbilt Department of Animal Care. A TOPAZ order submission is essential to ensure adequate animal numbers are available on the protocol and to track shipments. The PI should select the Non-approved eCatalog for the vendor and choose both “male” and “female” mice. In the Order Notes, it is crucial to document that either sperm or embryos will be received from the respective institution for rederivation. The delivery date should be set for July 4th (or as close as possible if grayed out) of the following year, indicating a “waitlisted” animal. For inquiries regarding MTA’s, please contact the Office of Technology Transfer at 615-936-7585.
Once DAC approval is granted through the TOPAZ request, the PI should proceed with submitting an iLab service request.
The successful rederivation of cryopreserved sperm or embryos depends on many factors. Some of the major factors that influence rederivation efficiency are:
- Genetic background of the mice. Some strains have low recovery rates.
- History of the sample provided. Samples can be damaged during cryopreservation, during storage, and by shipment.
- Use of different protocols at different institutions. Protocols have improved greatly over recent years, but many samples were cryopreserved before that.
Successful rederivation of cryopreserved sperm or embryos hinges on various factors. Major determinants include the genetic background of the mice, sample history, and variations in protocols across institutions. Although VGER takes utmost precautions to maintain the integrity of cryopreserved samples and has extensive experience with different protocols, the exact number of mice or the genotype of resulting pups cannot be guaranteed.
All shipping, handling costs, and cage expenses for any produced animals will be billed to the requesting PI.