A New Approach to RAS Inhibition
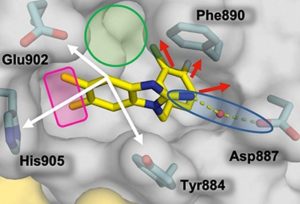
RAS is a small protein that acts as a molecular on/off switch to modulate numerous signaling pathways that control cell proliferation, differentiation, and survival. Activating mutations of RAS contribute to the malignant behavior of many kinds of cancers. Thus, there is a major ongoing effort to find drugs that block RAS function in the hope that they would be effective new cancer chemotherapeutic agents. This effort has been thwarted, however, by the fact that RAS lacks discrete binding pockets that can serve as a site of interaction for small molecule inhibitors. This led Vanderbilt Basic Sciences investigator Steve Fesik and his laboratory to target SOS1 (son of sevenless homologue 1) a protein that is required to activate RAS. They discovered several series of small molecules that promote the activity of SOS1 and increase RAS-dependent signaling at low concentrations. Paradoxically, at high concentrations, the most potent molecules lead to an overactivation of RAS signaling that triggers a negative feedback inhibition, suggesting that this may provide a novel approach to the blockade of RAS in cancer. To test their hypothesis, the investigators carried out a high-throughput screen of the Vanderbilt compound collection in search of new molecules that facilitate SOS1 activity. This led to the discovery of two dihydrobenzimidazoles that served as lead compounds for a structure-guided drug design campaign. In this approach, the researchers used X-ray co-crystal structures of selected compounds bound to a complex of SOS1 and RAS. The structural data provided key information regarding modifications of the molecules most likely to improve binding affinity. After multiple rounds of synthesis producing 63 new compounds, the investigators discovered molecules that bound to SOS1 with dissociation constants of <100 nM. Many of the more potent compounds were effective in modulating RAS activity in intact cancer cells, displaying activation at low concentration and suppression at high concentrations. Two of the compounds were the most potent yet observed in this cell-based assay. The findings provide the foundation for the further exploration of the potential of SOS1 activation to be used as a mechanism for inhibition of aberrant RAS-dependent signaling in cancer. The work is published in the journal The Journal of Medicinal Chemistry[T. R. Hodges, et al., (2018) J. Med. Chem., published online September 11, DOI: 10.1021/acs.jmedchem.8b01108]. Read More