 As a member of the class of ABC (ATP-binding cassette) transporters, the P-glycoprotein (P-gp) harnesses energy from ATP hydrolysis to power conformational changes that transfer substrates across the cell membrane against a concentration gradient. P-gp transports more than 200 structurally diverse substrates, thereby playing a role in the pharmacokinetics of numerous drugs and toxicants, and it is a key player in the ability of many cancer cells to resist the effects of anti-tumor drugs. Thus, the mechanism by which P-gp couples ATP hydrolysis to substrate transport is of great interest, leading Vanderbilt Basic Sciences investigator Hassane Mchourab, his collaborator Robert Nakamoto (U. of Virginia), and their laboratories to address this question using double electron-electron resonance (DEER) spectroscopy.
As a member of the class of ABC (ATP-binding cassette) transporters, the P-glycoprotein (P-gp) harnesses energy from ATP hydrolysis to power conformational changes that transfer substrates across the cell membrane against a concentration gradient. P-gp transports more than 200 structurally diverse substrates, thereby playing a role in the pharmacokinetics of numerous drugs and toxicants, and it is a key player in the ability of many cancer cells to resist the effects of anti-tumor drugs. Thus, the mechanism by which P-gp couples ATP hydrolysis to substrate transport is of great interest, leading Vanderbilt Basic Sciences investigator Hassane Mchourab, his collaborator Robert Nakamoto (U. of Virginia), and their laboratories to address this question using double electron-electron resonance (DEER) spectroscopy.
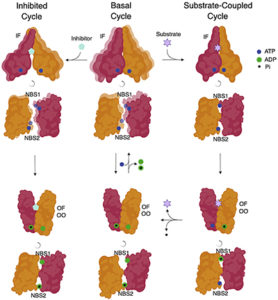
In the DEER approach, researchers engineer two spin label probes into the protein of interest, placing the probes at points in the protein that are expected to move with respect to one another during conformational changes. By constructing a number of molecules bearing probes at different locations, the investigator can glean considerable information about protein structure and dynamics. Prior work had suggested that during its transport cycle, P-gp hydrolyzes two molecules of ATP in distinct nucleotide binding sites (NBS1 and NBS2) in a defined order, and that the second hydrolysis event was coupled to release of bound substrate into the extracellular milieu.
However, recent structural data obtained from Cryo-EM studies had called this model into question, suggesting that ATP hydrolysis occurs after substrate release. In their new studies, the investigators used DEER to show that, in the presence of bound substrate, P-gp develops a high energy transition state during ATP hydrolysis that is distinguished by asymmetry between NBS1 and NBS2. In fact, the ability of a substrate to induce this asymmetry between the nucleotide binding sites was directly related to its ability to stimulate ATP hydrolysis by P-gp.
These findings indicated that substrate must be present while ATP is hydrolyzed, ruling out the possibility that ejection occurs first as suggested by the cryo-EM data. They also expanded their studies to include conformational changes that result during inhibitor binding, demonstrating that inhibitors lock P-gp into a symmetrical state that enables ATP hydrolysis in the absence of effective transport. The results provide the framework for a more highly refined model of P-gp function and will serve as the foundation for the design of better transport inhibitors that can be used to prevent the development of P-gp-mediated drug resistance during cancer chemotherapy. The work is published in the journal Science [R. Dastvan, et al., (2019) Science, 3s54, 689].