The WNT family of signaling proteins plays an important role in the regulation of cell proliferation, differentiation, motility, and migration via multiple pathways that are frequently dysregulated in cancer. The well-known canonical WNT pathway, critical to growth and development, functions primarily through modulation of the transcription factor β-catenin. The WNT-PCP (planar cell polarity) pathway regulates tissue polarity and cell motility through its influence on actin dynamics. The protein disheveled 2 (DVL2) is common to both WNT pathways, leading Vanderbilt Basic Sciences investigator Jason MacGurn and his collaborator *Donna Webb (Biological Sciences) to wonder how its activity is distributed between the two. Insight into this question came from their studies of the protein WWP1, a member of the NEDD4 family of E3 ubiquitin ligases known to play a role in membrane trafficking and cell signaling. E3 ubiquitin ligases transfer the protein ubiquitin to lysine residues of a target protein, leading ultimately to degradation of the target by the proteasome or to modulation of its functions and interactions.
The researchers discovered that WWP1 associates with DVL2 and also with USP9X, a deubiquitylating enzyme that removes ubiquitin groups from proteins. Further studies demonstrated that DVL2 is a substrate of both WWP1 and USP9X and identified four of DVL2’s lysine residues that appear to serve as targets for WWP1-mediated ubiquitylation.
Cells deficient in USP9X as a result of genetic manipulation demonstrated a suppression of canonical WNT pathway signaling. These cells also exhibited a decrease in the quantity and increase in the ubiquitylation of DVL2. Furthermore, USP9X deficiency led to a reduction of DVL2’s association with other proteins involved in the canonical WNT pathway. The researchers found that suppressing the expression of WWP1, or manipulations of the DVL2 protein that prevented its ubiquitylation reversed the loss of canonical WNT signaling associated with USP9X deficiency.
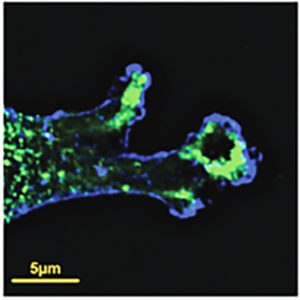
These results suggested that ubiquitylation of DVL2 by WWP1 prevents it from participating in canonical WNT signaling, whereas removal of ubiquitin groups from DVL2 by USP9X promotes that pathway. Further work revealed that DVL2 associates with a number of proteins involved in WNT-PCP-mediated signaling, and that USP9X deficiency promotes those associations. USP9X deficiency also led to increased cell migration and the localization of DVL2 in F actin-rich projections of the migrating cells, consistent with the hypothesis that ubiquitylation of DVL2 promotes its association with and signaling via the WNT-PCP pathway.
Together, the results suggest that WWP1 works together with USP9X as a rheostat to control the level of ubiquitylation of DVL2. WWP1-dependent ubiquitylation of DVL2 promotes its degradation and dissociation from proteins involved in canonical WNT signaling in favor of proteins involved in the WNT-PCP signaling pathway. USP9X-mediated deubiquitylation of DVL2 has the opposite effect. Clearly, both outcomes are important for growth, differentiation, and development, and derangements in either WNT signaling pathway can impact cancer by promoting cell growth or cell migration and metastasis. The work is published in the Journal Cell Reports [C. P. Nielsen, et al., (2019) Cell Rep., 28, 1074-1089].
* Donna Webb passed away in 2017 but collaborated on this project before her death.