Microtubules – protein polymers consisting of heterodimers of α- and β-tubulin – play a critical role in cell division, morphology, and intracellular transport. During microtubule polymerization, the tubulin heterodimers, each monomer of which contains a bound GTP molecule, align themselves head-to-tail into a multi-stranded structure containing two distinct ends: a plus-end exposing β-tubulin, and a minus-end exposing α-tubulin.
Over time, the GTP in the β-tubulin subunit is hydrolyzed to GDP, and when this occurs in all of the heterodimers at the growing tip of the microtubule, destabilization and rapid depolymerization quickly follow in a process known as catastrophe. These changes are particularly robust at the plus-end, so it has been the focus of most prior research. However, minus-ends are subject to polymerization/depolymerization cycles, leading Vanderbilt Basic Sciences investigator Marija Zanic, her collaborator Ryoma Ohi (University of Michigan), and their laboratories to explore minus-end dynamics in detail.
The researchers used total internal reflection fluorescence (TIRF) microscopy to simultaneously monitor microtubule dynamics at both ends in vitro. They discovered that the rate of microtubule polymerization increased with increasing tubulin heterodimer concentration, and that at any given concentration, the rate was faster at the plus-end than the minus-end. The frequency of catastrophe decreased with increasing tubulin heterodimer concentration (and polymerization rate), but when compared at concentrations that gave equal polymerization rates, the frequency of catastrophe was 4-fold higher at the plus- than the minus-end.
Using a fluorescently labeled protein that binds selectively to the GTP-tubulin cap at the growing microtubule tips, the researchers showed that when allowed to grow at equal rates, the caps at the two ends were the same lengths at the time of catastrophe. Thus, the differences in catastrophe frequency could not be explained on the basis of differences in the lengths of the stabilizing caps.
The researchers next used microtubules generated with tubulin heterodimers containing the non-hydrolyzable GTP analog GMPCPP so that they could study tubulin association and dissociation rates in the absence of catastrophe. They found that dissociation rate constants were lower at the minus-end than the plus-end. This suggested that dissociation rate differences might explain the distinct frequencies of catastrophe at the two ends.
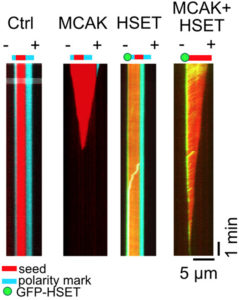
To further test this hypothesis, the researchers incubated their GMPCPP microtubules in the presence of the kinesin proteins MCAK and HSET. They found that MCAK caused a rapid depolymerization of the microtubules at both ends, whereas HSET accumulated at the minus-end, but had no major effect on depolymerization rates. Of greater interest, though, was the observation that HSET selectively protected the minus-end from MCAT-mediated depolymerization. Furthermore, HSET markedly reduced the frequency of minus-end catastrophe in microtubules formed from GTP-containing tubulin, whereas MCAK increased the frequency at both ends.
These data demonstrate the importance of differences in the GTP tubulin dissociation rate as a key determinant of the distinct dynamics between the two ends. Such accumulating insight is key to fully understanding the function of these critical drivers of so many important cellular processes. The work is published in the Journal of Cell Biology [C. Strothman, et al., (2019) J. Cell Biol., Published online August 16, DOI: 10.1083/jcb.201905019].