By Suneethi Sivakumaran
Human skin has three major layers: epidermis, dermis, and hypodermis. Epidermis is the outermost layer of the skin and serves as a waterproof protective barrier against pathogens and debris. At least 50 gene mutations can lead to defects in this layer and cause ichthyoses, a group of heterogeneous diseases that affect most or all of the skin. These genetic mutations affect the proteins, lipid metabolism, and assembly that are necessary for skin barrier function. A collaboration between the lab of Alan Brash (Pharmacology) and researchers in several Japanese institutions describes the role that a mutation in SDR9C7 has on the synthesis and metabolism of epidermal lipids in a recent publication in The Journal of Clinical Investigation.
The stratum corneum is the outermost layer of the five epidermal sublayers. The cell membrane of each of the cells in the stratum corneum is made up of a matrix of cross-linked proteins called the cornified cell envelope, and is surrounded by a lipid layer made of ceramides, cholesterol, and free fatty acids. This corneocyte lipid envelope, or CLE, is essential for the permeability barrier, and its absence causes a major structural defect in skin diseases associated with a dysfunctional skin barrier.
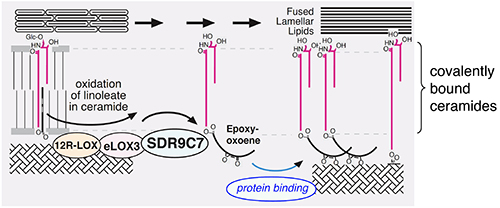
A major component of the CLE is a ceramide called esterified omega-hydroxy sphingosine (CerEOS), whose esterified component is linoleate fatty acid. Oxidation of linoleate in CerEOS by lipoxygenases is required for its fusion to proteins in the stratum corneum and for the formation of the CLE. Defective genes involved in the synthesis or metabolism of this ceramide leads to autosomal recessive congenital ichthyosis, a skin disorder characterized by dry skin, scaling, and skin thickening. However, the mechanism by which the oxidized ceramide gets fused to the stratum corneum has not been fully understood.
Recent investigations have uncovered mutations in a gene called SDR9C7 (short chain dehydrogenase/reductase family 9C member 7) in patients with autosomal recessive congenital ichthyosis. Although SDR9C7 is highly expressed in the epidermis, little is known about it beyond the fact that it is involved in vitamin A metabolism and that it is mutated in some cancers. The Brash team and their collaborators used biochemical and structural analyses in ichthyosis patient epidermises and mouse models to understand the role of SDR9C7 in skin barrier formation. They developed an SDR9C7 knockout mouse model that showed that mice lacking the protein had defective or no CLE in their epidermises.
In the SDR9C7 knockout mice, acylceramides related to the lipoxygenase pathway were abundant although other esterified acylceramides were absent. The knockout mice also lacked the protein products and ceramides required for the barrier function of epidermis. Patients with SDR9C7 mutations and the mouse knockout model both showed loss of lipid binding to the proteins in the epidermis. The skin of knockout mice showed normal expression of CerEOS, indicating that its biosynthesis was not affected. The authors also showed that recombinant SDR9C7 catalyzed the oxidation of the lipoxygenase products esterified in CerEOS, generating a derivative that bound directly to the proteins in the epidermis.
Collectively, the findings of this study suggest that lipoxygenases and SDR9C7 are critical in producing acylceramides with reactive ketone moieties. As these ketones are known to non-enzymatically couple to protein, the results suggest a mechanism for how ceramides such as CerEOS bind to the protein matrix and help solidify the skin’s protective barrier. This paper represents a major step forward in understanding the biochemical process of skin barrier formation and pathogenesis.
This research was supported by funding from the Advanced Research and Development Programs for Medical Innovation (AMED-CREST), the Agency for Medical Research and Development (AMED), the Japan Society for the Promotion of Science (JSPS), the Mochida Memorial Foundation for Medical and Pharmaceutical Research, the Uehara Memorial Foundation, and the Kanae Foundation for the Promotion of Medical Science.