By Wendy Bindeman

Sheila Collins, who is a professor of medicine and has a secondary appointment in molecular physiology and biophysics, first author Fubiao Shi, a postdoctoral fellow in the Collins lab, and colleagues have recently identified the transcription factor PPARγ as a novel regulator of natriuretic peptide “clearance” receptor C, which plays an important role in the metabolism of fat cells. Their findings were published in August 2021 in the Journal of Biological Chemistry.
We sat down with Shi and Collins to learn more about this exciting new research.
What problem does your research address?

The natriuretic peptide “clearance” receptor C, or NPRC, is an important regulator of a family of heart hormones known as “natriuretic peptides,” which have many roles in controlling blood pressure and adipocyte—fat cell—metabolism. In obese individuals, as well as in laboratory animal models of obesity, the level of NPRC in the adipose tissue is greatly increased, which interferes with the beneficial metabolic functions of the natriuretic peptides on fat cell metabolism and body weight. However, the molecular basis for how NPRC gene expression is regulated is still poorly understood.
What was unique about your approach to the research?
We used both a fat cell culture model and high-fat feeding in mice in combination with molecular biology approaches to investigate the transcriptional regulation of NPRC gene expression.
What were your findings?
We identified a transcription factor called PPARγ, which stands for peroxisome proliferator-activated receptor gamma, as a significant regulator of NPRC gene expression in mouse adipocytes, and established a new connection between PPARγ and the control of adipocyte NP signaling in obesity. PPARγ is also the target of an anti-diabetes drug, as diabetes is a common metabolic consequence of obesity.
Where is this research taking you next?
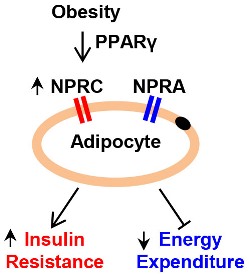
For the next step, we will investigate the regulation of NPRC gene expression in physiological conditions such as in brown adipose tissue and during adaptive thermogenesis. Brown adipose tissue or ‘brown fat’ consumes many calories instead of storing them in fat tissue and thus helps prevent body fat gain. Brown fat also appears beneficial for consuming glucose and thus can reduce the risk of type 2 diabetes. In newer work related to this publication we are starting to screen for molecules that could block the interaction of NPs with NPRC and, in effect, “inhibit the inhibitor.”
What are the societal/environmental/economic benefits of this research?
This study suggests that interventions that reduce NPRC level or activity could be metabolically beneficial for the treatment of obesity.
Funding
This work was supported by funding from the National Institutes of Health and the American Diabetes Association.
Go Deeper
The article “Diet-dependent natriuretic peptide receptor C expression in adipose tissue is mediated by PPARγ vialong-range distal enhancers” was published in the Journal of Biological Chemistry in August 2021.