By Olivia Trani
This article was originally published on the Oak Ridge National Laboratory website and was adapted with permission for publication at Vanderbilt University.
Water plays several important roles within the human body, even affecting the DNA in our cells. The entire surface of a DNA double helix is coated in layers of water molecules. This sheath of water attaches to the genetic material through hydrogen bonds, made by sharing hydrogen atoms between molecules. Through hydrogen bonds, water can influence how DNA takes shape and interacts with other molecules. In some cases, water can help proteins recognize DNA sequences.
Scientists can estimate where hydrogen bonds occur and how hydrogen atoms are shared, but it is difficult to gather experimental evidence. A research team led by Vanderbilt University has used a method that successfully captured the most detailed view to date of water’s hydrogen bonding patterns around DNA, opening new possibilities for studying how water impacts DNA function. Details on the methodology and the results, produced in part through neutron scattering at the Department of Energy’s Oak Ridge National Laboratory, are published in the journal Nucleic Acids Research.
“Water serves as a mediator between DNA and other molecules, even for very specific interactions. Before any molecule can bind to a segment of DNA, it must first go through this water shell,” said Martin Egli, professor of biochemistry and corresponding author of the study. “To advance our understanding of DNA processes, it’s important to know exactly what the surrounding water does and how it arranges itself around molecules.”
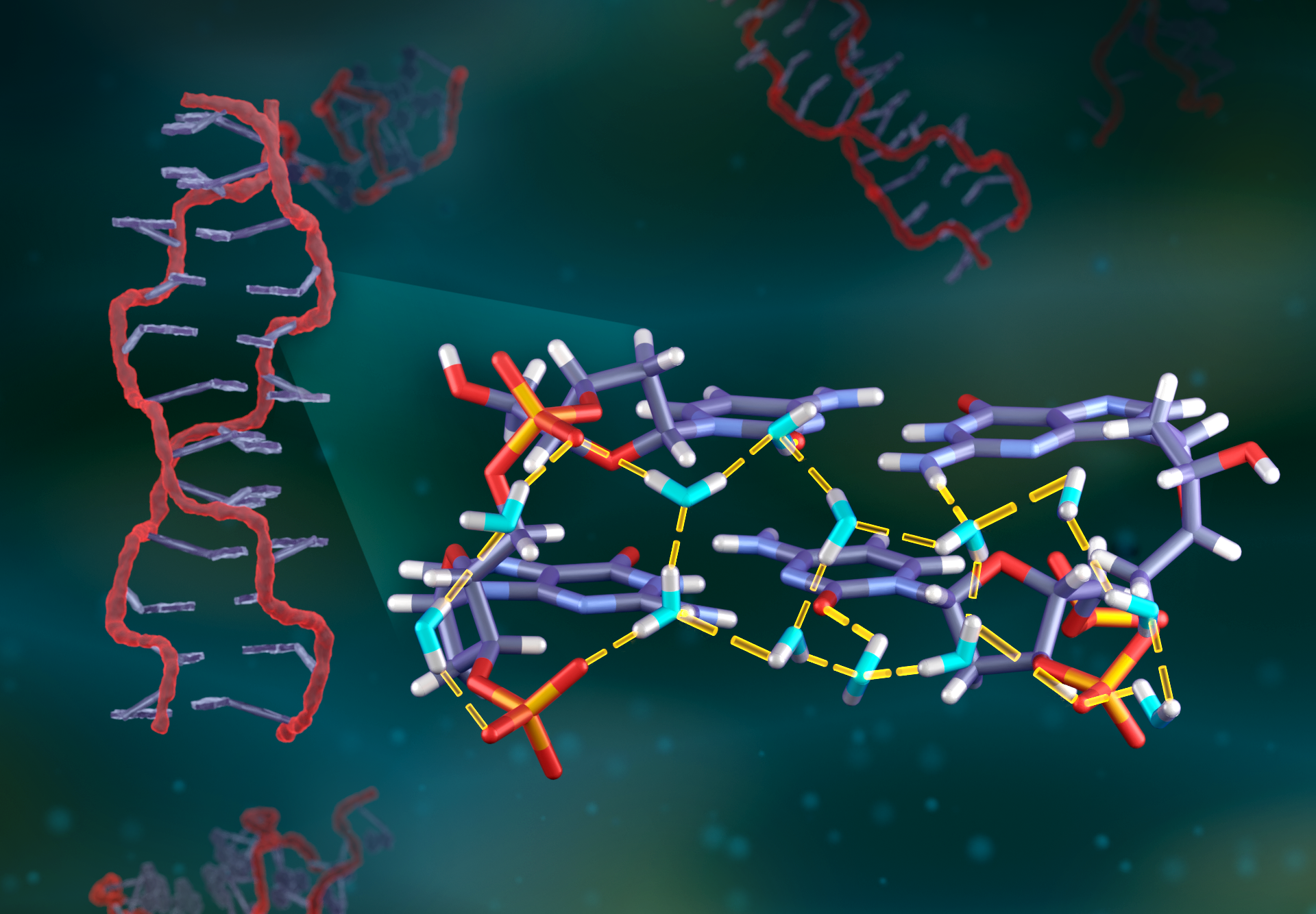
X-ray diffraction experiments have shed light on where water molecules are located around DNA, but the hydrogen bonding patterns between these molecules have remained hidden. Neutrons, on the other hand, are more sensitive to light elements, like the hydrogen atoms in water, which enable researchers to determine where hydrogen bonds occur and from which molecules they originate.
“With x-rays, the typical electron density you get for a water molecule is a sphere, like a soccer ball. You cannot see hydrogen atoms, so the molecule has no directionality to it,” said Leighton Coates, an ORNL scientist involved in this study. “Whereas, with neutrons, water molecules look more like boomerangs. You can see how the hydrogens are oriented and determine hydrogen bonding patterns.”
To conduct this research, the team used a crystalized sample of a well-studied DNA fragment with six base pairs, alternating between cytosine and guanine. Known as d(CGCGCG), this fragment was the first DNA sequence to have its crystal structure determined in 1979. Using a deuterium oxide solution, the scientists replaced many of the hydrogen atoms in the fragment with deuterium atoms. Deuterium, an isotope of hydrogen, is “seen” differently by neutrons compared with hydrogen, allowing the researchers to use deuterium to selectively collect information on the DNA and water structures.
The research team collected neutron diffraction data on this fragment using the macromolecular neutron diffractometer at ORNL’s Spallation Neutron Source, an accelerator-based neutron source facility that provides pulsed neutron beams. To reduce water movement, the team cooled the sample to 100 degrees Kelvin (nearly –280°F) using cold nitrogen gas.
“By lowering water mobility in our sample, we can keep the water molecules in a lattice-like arrangement, allowing us to lock down where they are and how they are positioned,” said Egli. “If we collected this data at room temperature, the positions of many water molecules would essentially be smeared, distributed over various locations in space.”
“With neutrons, we could also differentiate water molecules by the number of hydrogen bonds, such as whether they are involved in multiple bonds or just one,” added Joel Harp, research assistant professor of biochemistry and study co-author.
X-ray diffraction experiments were performed on a similar crystal at the Biomolecular Crystallography Facility at the Center for Structural Biology to determine where the oxygen atoms of water molecules are situated around the DNA fragment.
By combining these complementary techniques, the researchers achieved the most detailed analysis yet of water molecule orientations around a DNA double helix. They captured the orientations of 64 water molecules either in direct contact with the DNA fragment or nearby. The study revealed how hydrogen bonds are donated or accepted by water molecules within prominent parts of the DNA structure, including inside its grooves and around its sugar-phosphate backbone. Some of the hydrogen bonds were unexpected, going against previous assumptions, demonstrating that this method could help verify molecular dynamics models for DNA water networks.
The research team is now using this method to study how water behaves around other macromolecules, such as RNA.
“Now, I believe it’s time to apply what we have learned in more challenging projects,” said Egli. “Water is such a basic entity of life, and there are still many things to be discovered.”
This research was supported by the DOE Office of Science and the National Institutes of Health.
SNS is a DOE Office of Science user facility. UT-Battelle LLC manages ORNL for the DOE Office of Science. The Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, please visit http://www.energy.gov/science.