A new study led by Terunaga Nakagawa, professor of molecular physiology and biophysics and affiliate faculty of the Center for Structural Biology, is shedding light on our understanding of the molecular origins of some forms of autism and intellectual disability.

The collaborators, which include a team from McGill University, captured atomic resolution images of the fast-moving ionotropic glutamate receptor as it transports calcium for the first time. iGluRs and their ability to transport calcium are vitally important for many brain functions such as vision or other information coming from sensory organs. Calcium also brings about changes in the signaling capacity of iGluRs and nerve connections, which are key cellular events that facilitate our ability to learn new skills and form memories.
“Despite the importance in elucidating brain physiology and designing therapeutics that could control synaptic activity, the structural basis for ion permeation in AMPARs remains elusive,” Nakagawa said, referencing a specific iGluR. “The current study addresses the fundamental question about how ions permeate through glutamate receptor ion channels. It revealed unexpected regulation of AMPA type glutamate receptors by calcium ions.”
Nakagawa made extensive use of Vanderbilt’s Cryo-Electron Microscopy Facility to get the highest-resolution structures of iGluRs. Snapshots of the iGluR protein as it transports calcium showed a temporary pocket that traps calcium on the outside of the protein. With this information at hand, the researchers then used high-resolution electrophysiological recordings to detect the protein in motion as it transported calcium into the nerve cell. The biological mechanism discovered is not only conserved amongst all species of mammals but is also found in organisms that branched away from the evolutionary pathway of humans more than 500 million years ago.
“Visualizing the tiny ions and water molecules in the channel pore using cryo-EM technology was quite an amazing experience. It highlighted an ancient calcium binding pocket which we were able to understand further from a functional perspective in collaboration with the [Derek] Bowie lab [at McGill],” Nakagawa said. “Our finding is fundamental to calcium signaling in neurons and raises interesting hypotheses about synaptic function that could be tested by experiments in the future.”
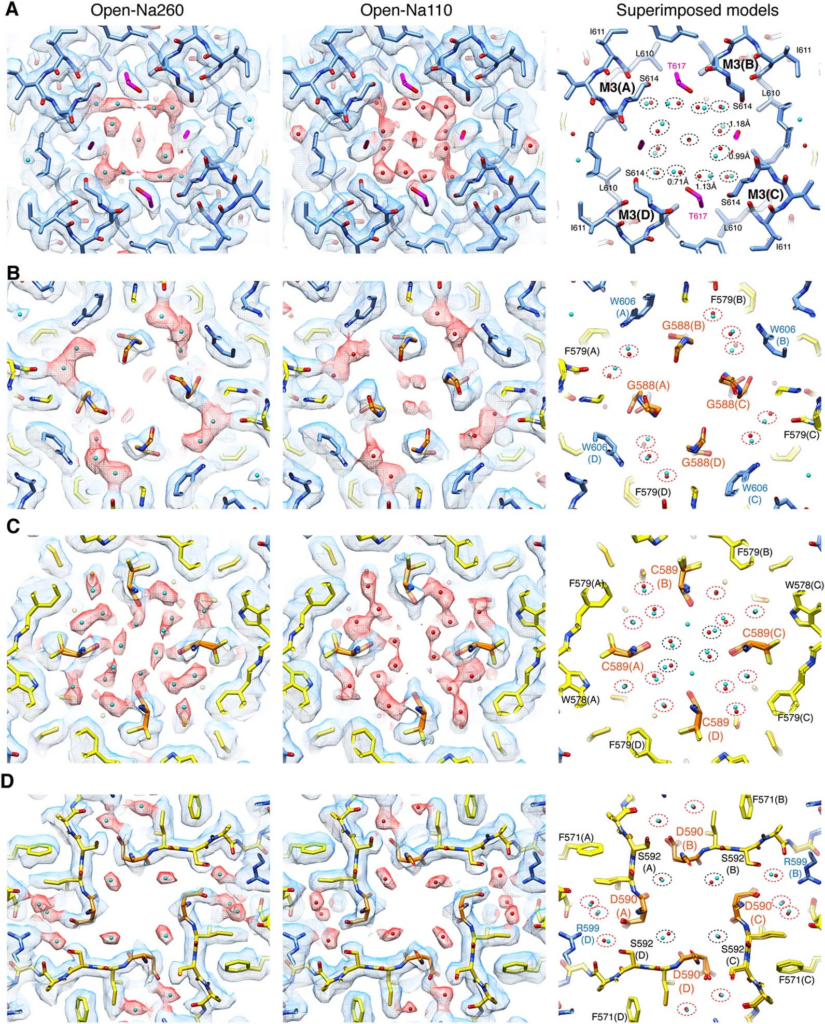
Derek Bowie, co-director of the Cell Information Systems group in the School of Biomedical Sciences at McGill, added that “the results are important because we describe for the first time the mechanism by which calcium is transported, which ultimately drives the cellular processes that lead to learning and memory.”
The work was supported by the National Institutes of Health, the Stanley Cohen Innovation Fund, and the Canadian Institutes of Health Research.
GO DEEPER:
The article “The open gate of the AMPA receptor forms a Ca2+ binding site critical in regulating ion transport” was published in the journal Nature Structural and Molecular Biology on Feb. 26, 2024.