When I was 13, my brother Scott (12) and I joined our neighbors Matha (12) and Jay (10) on a trip to their family cottage on the bank of the Allegheny River in western Pennsylvania. I had fantasized about this trip for months; I was fixed on the notion that when we got there I would build a raft and float down the Allegheny River like Huckleberry Finn, preferably accompanied by my reliable ally in the war on little brothers, Matha.
Although I did construct a raft by tying some logs together, the cruel river gods of the Allegheny had other plans for me. Upon launching said raft, it promptly sank and broke up when swept into underwater rocks. Scott then accidentally dropped a boulder on Matha’s toe, who had to go to the emergency room in the nearest town to get stitched up. In the rippling of the waters of the Allegheny I could hear the river nymphs. Oh, how they laughed and laughed…!
My interest in rafts was re-kindled by my fascination with membrane phenomena, leading to a career of research on membranes and membrane proteins. During the ’80s and ’90s, a new membrane phase was coming to be appreciated—the liquid ordered (Lo) phase. This phase was most often seen to be formed by hydrated lipid mixtures containing cholesterol and a phospholipid with relatively long and fully saturated tails (often a sphingolipid). The dynamics of this phase are intermediate between the fluid “liquid disordered” (Ld) phase, formed by a glycerophospholipid containing short chains and/or at least one cis double bond on one or both of its tails, and the quasi-2D-crystalline solid phase (So), formed by saturated phospholipids. A key feature of the Lo phase is that its lipids are more stiff than in the disordered phase (even approaching the very high order of the solid phase) but still undergo rapid rotational and 2D translational diffusion in the bilayer plane.
In the late ’90s the concept of lipid rafts was floated. Lipid rafts were hypothesized to be domains with Lo-like properties present in biological membranes and were postulated to be ensembles of lipids and proteins that cluster together and diffuse as a raft-like unit through the surrounding 2D fluid bilayer. Around the same time, microscopy was used to directly visualize Lo phase domains in certain cholesterol-containing ternary mixtures as circular spots floating in an Ld background, unambiguously confirming the presence of phase demixing. Soon after, ternary phase diagrams for cholesterol-containing mixtures were mapped, confirming that certain compositions in certain temperature ranges are populated by co-existing Lo and Ld phases. These studies confirmed that the lipid raft hypothesis was physically reasonable, supporting the plausibility of lipid rafts in biological membranes. Scientists leading this early and important body of work include Kai Simons, Ken Jacobson, Deborah Brown, Erwin London, Fred Cohen, Sarah Keller, and Gerald Feigenson.
Experimental confirmation of the lipid raft hypothesis in actual biological membranes, where there are many hundreds of chemically distinct lipids and numerous membrane proteins, originally proved elusive. This led to years of controversy about whether lipid rafts existed in biology or whether the lipid raft hypothesis was just plain wrong. This editorial highlights the discoveries and concepts that I personally find to be compelling as evidence that the lipid raft hypothesis is essentially correct, but in ways that are both more nuanced and have more profound biological implications than as originally conceived.
The evidence
It is possible to bleb off “giant plasma membrane-derived vesicles” from living mammalian cells, a process that typically also involves exposing the vesicles to certain reagents, such as dilute formaldehyde and dithiothreitol. If these GPMVs are then labeled with a fluorescent dye that partitions preferentially into Lo-like membrane domains, no phase separation is observed at 37 ˚C using an ordinary fluorescence microscope. However, upon lowering the temperature, typically to about 10 ˚C below physiological temperature, fluorescence microscopy reveals the formation of micron-scale membrane domains, appearing as easily observed spots with well-rounded edges. This indicates that mammalian membranes have lipid and membrane protein compositions with a considerable potential to phase separate to form large Lo domains.
This is not surprising since the plasma membranes of most mammalian cells contain 25 mol%-or-higher levels of cholesterol. So, what happens when the temperature is raised back to the physiological temperature and the lipid rafts seemingly disappear? This is where critical phase theory comes into play, making powerful testable predictions about the nature of biomembranes, as well as offering a unifying conceptual framework for understanding the nature of lipid rafts. Over the past nearly 20 years, Sarah Veatch (University of Michigan) has championed the application of this theory to membranes, with Sarah Keller (University of Washington), Barbara Baird (Cornell University), and their acolytes also making very important contributions.
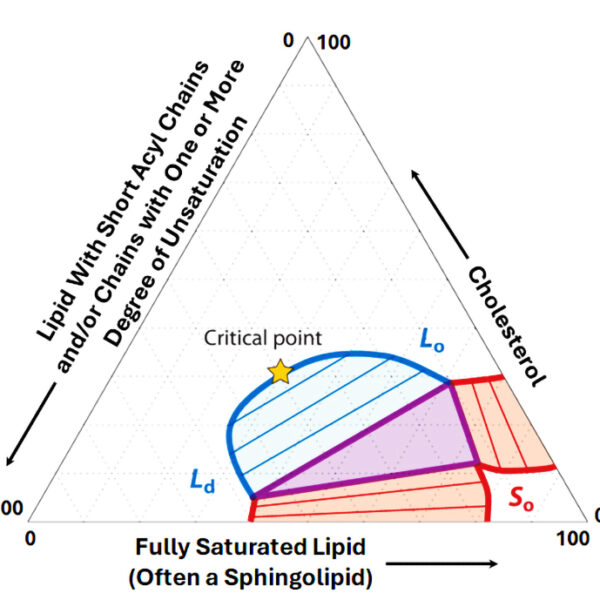
As noted above, some ternary phase diagrams for cholesterol-containing lipid mixtures include a region where the Lo and Ld phases coexist, meaning that the micron-scale lipid rafts are easily observed in this regime. At the boundary between the most cholesterol-rich composition of the co-existing phases and a phase that is effectively a homogeneous average of the Lo and Ld phases is a critical point (Figure 1). Near the critical point the Lo and Ld phases have the same energy and composition and “fluctuate” from one phase to the other as driven by random thermal (kBT) energy. The size of the critical fluctuations (the “correlation length”) can be much smaller (anywhere from microns very close to the critical point to tens of nanometers further away). Moreover, the shape of the fluctuations is not round with smooth edges, but irregular, heterogeneous, and dynamic. As either the temperature or the cholesterol content is increased, pushing the system past the critical point, the size of the fluctuations decreases until they reach molecular dimensions, at which point the lipid mixture uniformly populates the homogenous average phase alluded to above. The “2D Ising model” offers a quantitative description of the behavior at and near the critical point.
Using fluorescence microscopy, researchers saw that the 2D Ising model can be used to explain the phase behavior of both synthetic giant unilamellar vesicles (see Figure 2) and cell-derived GPMVs as a function of temperature. Well below physiological temperature, large and well-rounded ordered phase domains are easily visualized. The ordered phase domains shrink and take on jagged edges as the temperature is raised through the critical temperature (Tc), which is several degrees below the physiological temperature. Above Tc the fluctuations become small and irregular, finally appearing grainy as the resolution of the microscope is maxed out.
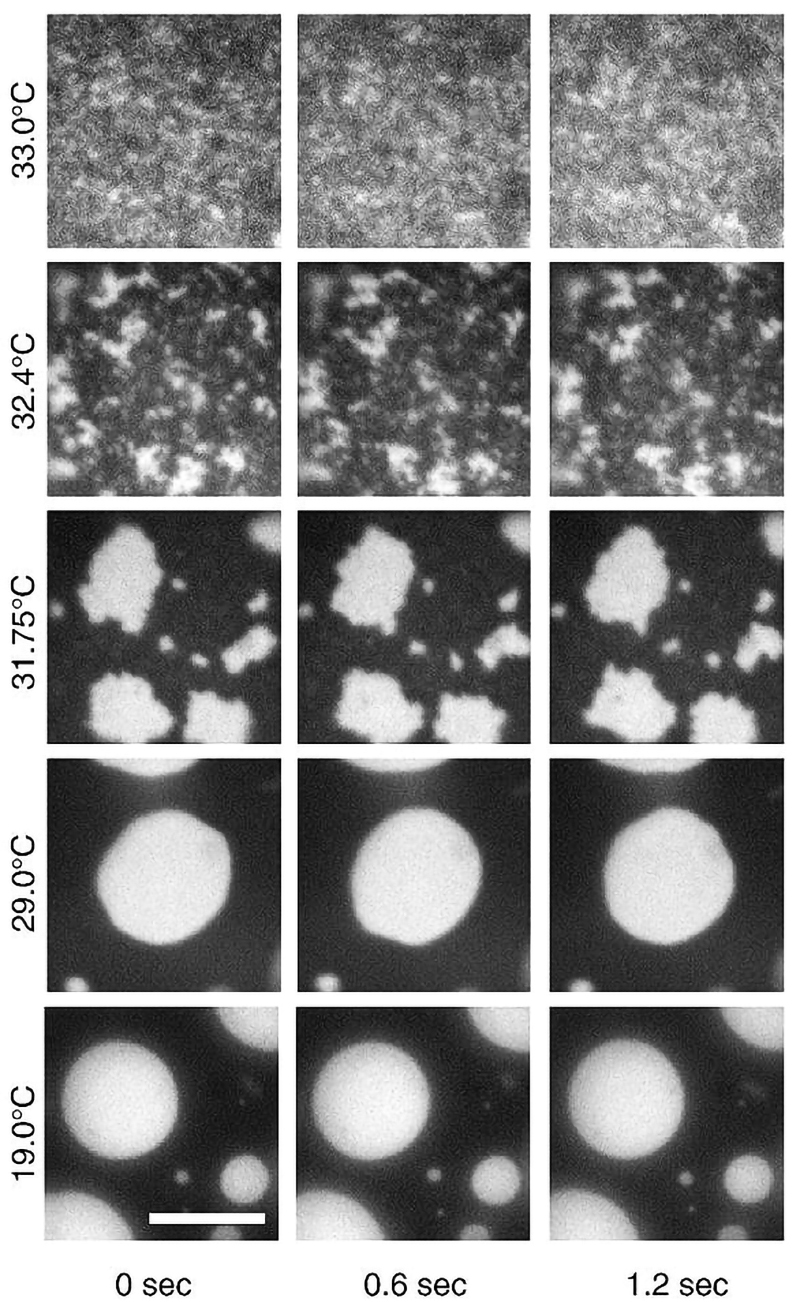
These observations are central to a body of work that strongly supports the notion that, at physiological temperatures, biological membranes have compositions that place them near critical points, with lipid compositions being tuned so that under default physiological conditions lipid rafts are present in the form of critical fluctuations, with correlation lengths on the scale of tens of nm (as has now been observed in mammalian cell membranes). Rafts are therefore most likely routinely present, but smaller, more irregular, and more dynamic than originally hypothesized.
The size of the fluctuations in biological membranes may often be on the same scale as membrane-interacting proteins and complexes. To the degree that the cell is able to tune the scale and dynamics of membrane fluctuations, the fluctuations may represent more than just a complex aspect of bilayers as a solvent for membrane proteins. Rather, they may even provide a regulatory mechanism for modulating protein localization and trafficking, protein-protein interactions, structure and dynamics, and function. One wonders not only if such cellular tuning of critical behavior is an evolved trait of eukaryotic cells, but also, if so, whether there could be different scales of such regulation ranging from local to global, and from fast to slow. This is an almost completely unexplored frontier.
It is also the case that lipid and membrane protein compositions of phases near the critical point can be thought of as adaptive fluids that have a high “compositional susceptibility.” What this implies is that when extrinsic factors (defined below) interact with critical fluctuations there is a high potential for significant local changes in the size of the fluctuations, leading to transitions from a fluctuating critical phase to larger and locally stable raft-like assemblies that may, for example, serve as sites for focal adhesion, cell junctions, platforms for intracellular signaling complexes, or cytoskeletal connection sites. Order from seeming chaos! The extrinsic factors that could trigger such changes include changes in temperature (as could occur during burns, hypothermia, or fever), ligand-stimulated complex formation between membrane proteins, anesthetics, a change in the transmembrane electrical potential, or the presence of certain proteins (such as palmitoylated proteins, cytoskeletal anchors, or raft-favoring membrane proteins).
A generally applicable principle is that to support life, nature can and will exploit almost any phenomenon that is physically and chemically feasible. Critical phase theory describes one such phenomenon. It appears to offer a satisfying resolution to the lipid raft controversy. Could it be that some other order-out-of-chaos phenomena in biology also reflect systems that are set up to fluctuate near a critical point under default conditions? A quick literature search shows that this question has already crossed the minds of others. In any case, I encourage you to check out critical phase theory for yourself! One hopes the water gods and goddesses will smile on approvingly.
Acknowledgements I thank Drs. Sarah Veath, Katherine Stefanski, and Anne Kenworthy for helpful comments and edits. Sources for this article and additional reading about critical phase theory as applied to biological membranes can be found in this link. I wish you all a pleasant start to autumn or, as applicable, spring!