Melanoma is the most common form of skin cancer, but for patients with advanced melanoma who no longer respond to standard immunotherapy, treatment options are painfully limited. Vanderbilt researchers led by Professor Emerita of Pharmacology Ann Richmond have discovered a promising new drug combination that targets three key pathways at once and that may help these treatment-resistant patients respond once again to the body’s own immune defenses. Their groundbreaking research, published in Frontiers in Oncology, described preclinical work but, as it used a combination of drugs that are already in clinical trials for other conditions, could have a faster path toward testing in people.

Melanoma affects more than 300,000 people worldwide, and the incidence of this type of cancer has been increasing in recent years. Metastatic melanoma tumors, which have spread from their origin to other parts of the body, will often develop resistance to immune checkpoint inhibitors. ICI are a form of immunotherapy that removes the molecular “brakes” preventing immune cells from attacking tumors and is the standard of care for metastatic melanoma. Yet, ICI are less effective for melanoma tumors in an environment that suppresses the immune system.
Targeted drugs to treat some metastatic melanoma patients exist, such as BRAF/MEK inhibitors, a combination targeted therapy for cancers with a specific mutation in their BRAF gene that block the MAPK signaling pathway that drives tumor growth. The combination therapy, which includes a BRAF inhibitor (like dabrafenib) and a MEK inhibitor (like trametinib), is more effective and prolongs survival compared to BRAF inhibitors alone, making it a standard of care for BRAF-mutant advanced melanoma when there is no response to ICI.
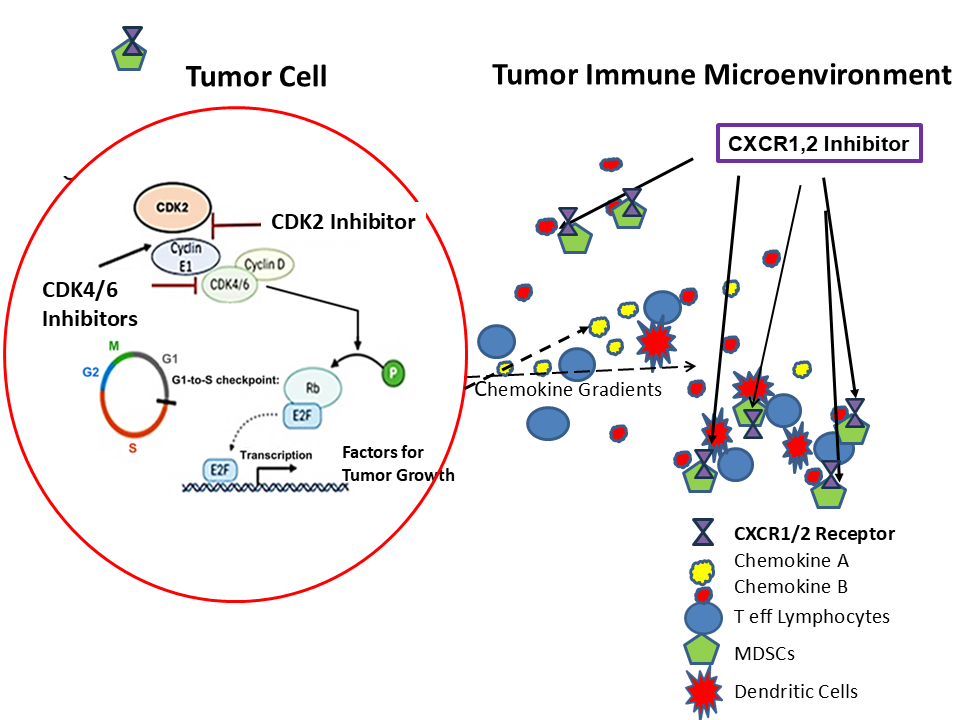
Targeted drugs like BRAF/MEK inhibitors help some patients, but options are especially scarce for those without BRAF mutations. A class of drugs called CDK inhibitors can slow tumor growth and boost the immune system, particularly when paired with other treatments, so researchers are exploring innovative combinations to try to address the problem. Another promising strategy targets immune-suppressing cells in the tumor environment with CXCR1/2 blockers, which can help the body’s T-cells fight cancer. Building on these findings and aiming to create a more powerful treatment for patients with resistant melanoma, scientists are now testing a combination of CDK inhibitors and CXCR1/2 blockers in mouse models.
“This is the first study combining both CDK4/6 and CDK2 inhibitors with a chemokine receptor antagonist to treat melanoma tumors,” Richmond said. Chemokine receptor antagonists are drugs that block certain proteins that normally act like “signals” to attract immune cells to diseased areas. “We hypothesized that adding CDK2 inhibitors would overcome resistance mechanisms for CDK4/6 inhibitors, and that CXCR1/2 inhibitors would interfere with the recruitment of immunosuppressive immune cells, ultimately resulting in a tumor microenvironment more receptive to immune challenge.”
Using preclinical mouse models, the study revealed three major outcomes:
- Response in untreatable tumors: Melanoma that did not have the common BRAF mutations and no longer responded to standard immunotherapy showed strong responses when treated with a combination of the three targeted drugs, CDK4/6, CDK2, and CXCR1/2 inhibitors.
- Double benefit: The treatment slowed tumor growth and boosted the body’s immune system to better fight melanoma.
- Immune system rebalanced: The drugs shifted the tumor environment to be more supportive of immune attack, increasing helpful T cells, reducing suppressive cells, and turning certain immune cells into tumor-fighting types.
Richmond emphasized that the translational promise of the tested drug combination was high and its path to human studies could be accelerated because all three drugs are already in clinical trials for other conditions. Future directions may include more preclinical testing in human melanoma tumors in organoid models or humanized mouse models or moving directly into a small clinical trial.
The Richmond lab’s approach represents a major step toward more effective therapies for treatment of immune therapy–resistant melanoma tumors with RB, BRAF, or NRAS mutations, Richmond said, potentially offering new hope for patients facing limited options today.
The study was the result of a collaborative effort across Vanderbilt and international partners. Vanderbilt scientists Jinming Yang, Weifeng Luo, Patricia Ward, and Chi Yan performed “outstanding work,” according to Richmond Yang and Luo made equal contributions as first authors. Richmond credited contributions from Syntrix Biosystems, the Pasteur Institute in Paris, and Sheau-Chiann Chen, who provided statistical expertise.
Go deeper
The paper “Combined treatment with CDK4/6, CDK2, and CXCR1/2 inhibitors effectively halts the growth of BRAF wild-type melanoma tumors” was published in Frontiers in Oncology in August 2025.
Funding
This research used funds from the National Cancer Institute and the Department of Veterans Affairs.
Open Access
The study was published open access through a transformative agreement negotiated by Vanderbilt University’s Jean and Alexander Heard Libraries. Transformative agreements eliminate traditional paywalls and remove the obstacle of article processing charges, ensuring immediate and unrestricted access to research worldwide. Vanderbilt authors can learn more about the Heard Libraries’ agreements supporting open access publishing in this research guide.