CK1 is required for a mitotic checkpoint that delays cytokinesis
CK1 is required for a mitotic checkpoint that delays cytokinesis
Alyssa E. Johnson, Jun-Song Chen, Kathleen L. Gould
Highlights
•Dma1’s FHA domain binds to a pTXXpS phosphomotif on Sid4
•CK1 is required for Dma1-dependent Sid4 ubiquitination
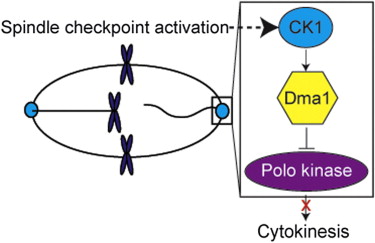
•CK1 phosphorylation of Sid4 is required for the Dma1-dependent mitotic checkpoint
•CK1 concentrates at SPBs and phosphoprimes Sid4 for Dma1-mediated ubiquitination
Summary
Failure to accurately partition genetic material during cell division causes aneuploidy and drives tumorigenesis [1]. Cell-cycle checkpoints safeguard cells from such catastrophes by impeding cell-cycle progression when mistakes arise. FHA-RING E3 ligases, including human RNF8 [2] and CHFR [3] and fission yeast Dma1 [4], relay checkpoint signals by binding phosphorylated proteins via their FHA domains and promoting ubiquitination of downstream targets [5]. Upon mitotic checkpoint activation, S. pombe Dma1 concentrates at spindle pole bodies (SPBs) in an FHA-dependent manner and ubiquitinates Sid4, a scaffold of Polo kinase, to suspend cytokinesis [6]. However, the kinase or kinases that phosphoprime Sid4 for Dma1-mediated ubiquitination are unknown. Here, we report that the highly conserved protein kinase CK1 transmits the signal necessary to stall cytokinesis by phosphopriming Sid4 for Dma1-mediated ubiquitination. Like Dma1, CK1 accumulates at SPBs during a mitotic arrest and associates stably with SPB components, including Sid4. Our results establish CK1 as an integral component of a mitotic, ubiquitin-mediated checkpoint pathway.