by Kathryn R. Brewer, Ph.D. candidate in biochemistry, Vanderbilt University

A twenty-year-old woman waits to see her gynecologist. For years she has been experiencing troubling symptoms – long menstrual cycles, excess facial hair, weight gain, and most recently, a diagnosis of prediabetes. She hopes that her physician will be able to help her manage her symptoms with a comprehensive diagnosis and intervention plan. Instead, what she gets is a cryptic diagnosis, a prescription for birth control, and a recommendation to lose weight, which she has been attempting for years with minimal success. Disheartened, the woman leaves the office with more questions than she came in with, all of which center around her newly diagnosed disorder – PCOS.
Stories like this one are unfortunately extremely common. Polycystic ovarian syndrome is a complex endocrinological disorder with widespread prevalence, affecting approximately one in ten women worldwide (2). Symptoms frequently include increased androgen levels (hyperandrogenism), infrequent or no ovulation, and physical ovarian cysts in some, but not all, cases (2, 6, 7). Patients with hyperandrogenism—accounting for 70% of all patients (2, 9)—have increased risk of additional health complications (9-11), including insulin resistance and type 2 diabetes (10, 12). Treatment for PCOS typically involves extensive lifestyle modification (12) and medications to help manage reproductive symptoms and insulin resistance (13), but there are no treatments that directly target hyperandrogenism. Because of this, women with PCOS often feel unsatisfied with the quality of their care and are belabored by the changes necessary to live out their daily lives (14).
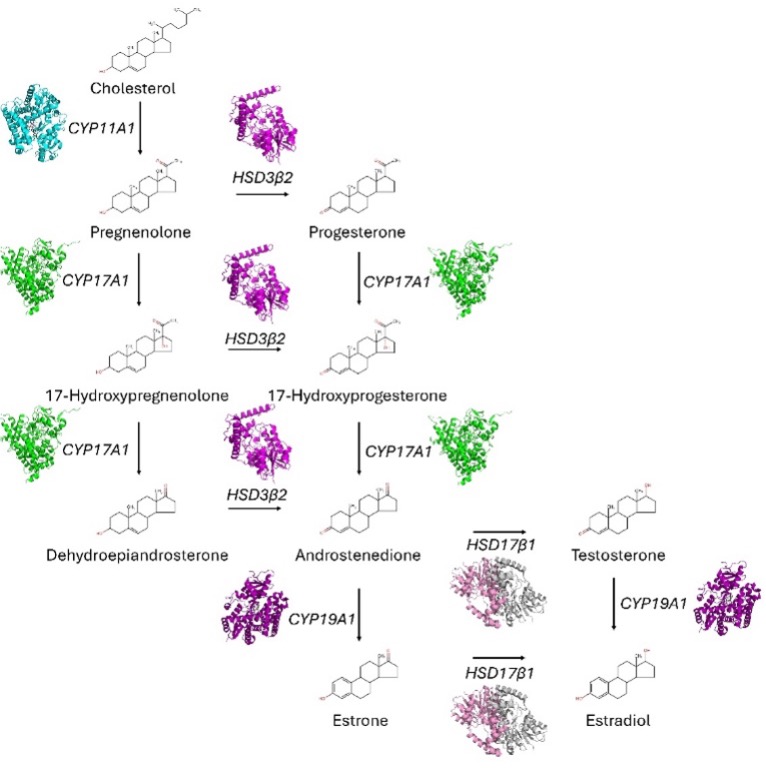
In an effort to identify molecular drivers of PCOS, multiple genome-wide association studies have been conducted (15), revealing that, like many endocrinological diseases, there is no one clear genetic target for PCOS treatment. However, analysis of primary ovarian theca cells from PCOS patients have provided strong phenotypic indicators of promising targets (16-20), including overexpression of mitochondrial steroidogenic enzymes CYP17A1, an enzyme with combined 17α-hydroxylase and 17,20-lyase activities for 17OH-progesterone and 17OH-pregnenolone (21, 22) as well as CYP11A1, the P450 sidechain cleavage enzyme that constitutes the first enzyme in the steroidogenic pathway (22) (Figure 1). Attempts have been made to decrease the activity of these enzymes indirectly using available anti-androgens (23), but have not yet shown satisfactory results. Additionally, anti-androgen use is controversial due to the potential for birth defects (24). Developing specific inhibitors for CYP11A1/CYP17A1 enzymes may thus be a more effective option for treating PCOS.
In June of this year, a Science paper (25) identified artemisinin, an FDA-approved drug currently used as a frontline treatment for malaria (26), as a targeted CYP11A1 degrader. This study was impressively thorough, using rat models, biochemical analysis, thermal shift assays of purified proteins, and a pilot clinical trial to demonstrate the drug’s efficacy and elucidate its mechanism of action. Artemisinin induced degradation of CYP11A1 by initiating its interaction with LONP1, a protease with a critical role in mitochondrial proteostasis (27). Artemisinin was predicted to bind in the proteolytic domain of LONP1, increasing LONP1-CYP11A1 interaction and bringing CYP11A1 in proximity for degradation. Excitingly, in a pilot clinical trial covering 19 PCOS individuals, artemisinin treatment resulted in improved hyperandrogenism and normalized patient menstrual cycles, with over 50% of patients recovering regular cycles.
This work has brought targeted PCOS therapy back into the forefront, sparking highlights in scientific journals (28-30) and news outlets. As this enthusiasm transitions to continued scientific inquiry, there are several considerations to keep in mind, both for the current drug candidate and additional therapeutic opportunities. In this short article, I hope to provide an overview of those considerations and possibilities.
Artemisinin gives new hope for PCOS treatment – and one major concern
These preliminary results certainly demonstrate promise for a new PCOS treatment. Additionally, clinical trials for other applications have validated artemisinin’s safety (31-33). However, as CYP11A1 is the first enzyme in steroid biosynthesis (Figure 1), the effect of artemisinin on downstream hormones is a major point of concern, particularly progesterone. Studies have demonstrated decreased progesterone levels (34-36) and progesterone resistance (37, 38) in PCOS, leading to increased risk of endometrial cancer (39) as well as recurrent pregnancy loss (40). Notably, the Science article noted that artemisinin treatment reduced progesterone levels in rat models, suggesting that such may also be the case in PCOS patients. As progesterone was not evaluated in their pre-clinical trial, the impact of artemisinin on progesterone production in humans should be examined.
Two birds, one stone? Utilizing CYP17A1 inhibitor research to advance PCOS treatment options
This study provided the first example of targeting a steroidogenic enzyme for treating PCOS, raising the possibility of inhibiting others. CYP17A1 is particularly attractive because it acts downstream to progesterone production (22) (Figure 1), indicating that it may inhibit androgen synthesis without reducing progesterone. Several CYP17A1 inhibitors have already been identified. Abiraterone acetate, which has FDA approval for prostate cancer in men (41-43), has also demonstrated androgen reduction in women in clinical trials (44, 45). However, abiraterone has significant side effects due to off-target activity (46, 47). Additionally, some clinical trials demonstrated adverse fetal outcomes (48). While this may be an option for PCOS treatment, these caveats should be considered. Notably, abiraterone analogs with increased CYP17A1 selectivity in vitro have been identified (49, 50), which may be attractive alternatives.
Several other candidates have also been identified, both in in vitro and cellular studies (51-57) as well as fragment-based in silico screens (58, 59), which cover a wide spectrum of chemical space (60). Of particular note are (+/-)-orteronel (61) and (S)-seviteronel (62), which have made it to clinical trials, providing preliminary evidence of efficacy and tolerance in women (63, 64). These may be especially attractive starting points for a potential PCOS therapy. It is also worth noting that the CYP17A1 active site is spacious and can accommodate a wide variety of ligands (60), providing an array of options for chemical modification of these already chemically diverse lead compounds. There is thus a great deal of potential for finding an effective inhibitor, making it worthwhile to explore and optimize these candidates for application in PCOS.
PCOS subtypes highlight the importance of tailored therapeutics as a component of PCOS treatment
Finally, as these and other targets are explored, an important consideration arises from the growing recognition of PCOS subtypes (65, 66). While once considered to be a single, phenotypically complex disorder, recent studies have revealed distinct groupings of PCOS patients, broadly separated into metabolic and reproductive categories. Importantly, these PCOS subtypes have unique gene expression (67), phenotypic outcomes (66), and comorbidity risks (68), indicating unique genetic causes and subsequently requiring tailored therapeutic strategies. While the targets described above are perhaps most applicable to women in the metabolic category (69, 70), evaluating drug effectiveness across PCOS subtypes will be a crucial element in determining broader therapeutic potential. Additionally, exploration of the pathogenic mechanisms that uniquely define these subtypes may also assist in the identification of subtype-specific targets and therapies.
Conclusion
The discovery of a novel PCOS therapeutic represents a major leap forward in this field, providing a new source of hope for researchers and patients alike. It is my hope that through this article, I have brought to light the need for scientific investment into this common but overlooked disease, as well as the various caveats to consider and hurdles to be overcome. Most importantly, however, I hope that I have conveyed the opportunities that lay before us, which are, in my opinion, plentiful. A new horizon is dawning in PCOS research. Let‘s take the time to explore it.
Acknowledgments
I would like to thank Chuck Sanders for his help in editing this article. I would also like to thank Basically Speaking for giving me the opportunity to present it to our community. This is a topic of great scientific and personal interest to me, and I am grateful to be able to share it with you all. The references for this article are here. A very similar version of this article is published in the Protein Society newsletter Under the Microscope.