cell reprogramming by forced Pdx1 expression
Context-specific α-to-β-cell reprogramming by forced Pdx1 expression
Yu-Ping Yang1,Fabrizio Thorel2,Daniel F. Boyer1,Pedro L. Herrera2 and Christopher V.E. Wright1,3
1Vanderbilt University Program in Developmental Biology, Department of Cell and Developmental Biology, Vanderbilt University Medical Center, Nashville, Tennessee 37232, USA;
2Department of Cell Physiology and Metabolism, University of Geneva Faculty of Medicine, CH-1211 Geneva 4, Switzerland
Abstract
Using single transcription factors to reprogram cells could produce important insights into the epigenetic mechanisms that direct normal differentiation, or counter inappropriate plasticity, or even provide new ways of manipulating normal ontogeny in vitro to control lineage diversification and differentiation. We enforced Pdx1 expression from the Neurogenin-3-expressing endocrine commitment point onward and found during the embryonic period a minor increased β-cell allocation with accompanying reduced α-cell numbers. More surprisingly, almost all remaining Pdx1-containing glucagon/Arx-producing cells underwent a fairly rapid conversion at postnatal stages, through glucagon–insulin double positivity, to a state indistinguishable from normal β cells, resulting in complete α-cell absence. This α-to-β conversion was not caused by activating Pdx1 in the later glucagon-expressing state. Our findings reveal that Pdx1 can work single-handedly as a potent context-dependent autonomous reprogramming agent, and suggest a postnatal differentiation evaluation stage involved in normal endocrine maturation.
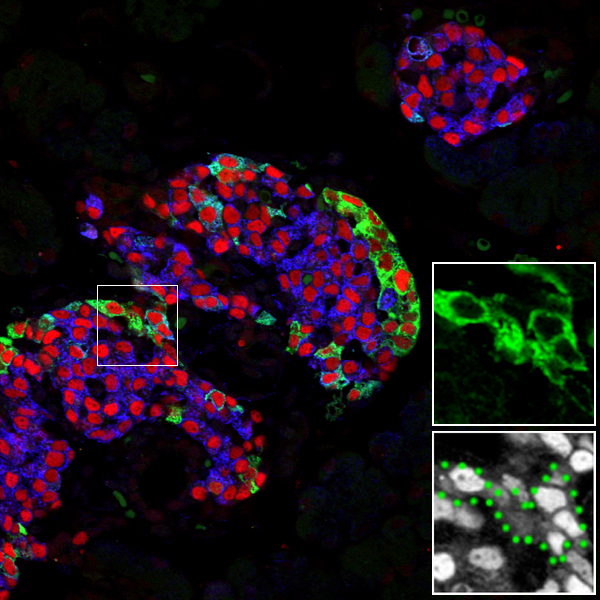
A major hurdle for cell replacement-based diabetes therapy is the difficulty of supplying vast numbers of functioning insulin-producing β cells. One method could be through the reprogramming of alternative cell types. While this process might be easier with closely lineage-related cells, even substantially different cells may be susceptible (e.g., Zhou et al. 2008).
Recent studies reveal significant plasticity between pancreatic α and β cells under certain induced conditions, implying a potential route to β cells through α cells. In a near-total β-cell destruction and regeneration model in adult mice, a proportion of new β cells were produced from α cells via a bihormonal glucagon+insulin+ (Gcg+Ins+) transitional state (Thorel et al. 2010). The interconversion presumably occurs in response to a combination of the physiological need to replenish β cells and regeneration-induced stress, raising questions as to the local or systemic signals triggered by such lesions. Direct superimposition of a pro-β-lineage condition was reported when Pax4 expression was forced in pancreatic or endocrine progenitors or in embryonic α cells to redirect endocrine differentiation or coax pre-existing α cells into β cells. The converted cells seemed similar to normal β cells and temporarily improved glycemia under induced diabetes, although the effect was superseded by uncontrolled α-cell neogenesis and fatality caused by extreme hyperglycemia (Collombat et al. 2009). These studies on the ability of a single lineage-allocating transcription factor to sustain complete cell fate conversion suggest that similar analyses for other transcription factors could be insightful. Determining which factors induce specific types of lineage reprogramming, as well as the repertoire of cellular competence states amenable to fate switching, could lead to pharmacological intervention to activate such factors in vivo, or to improved differentiation of embryonic stem cells to β cells.
Clues to the fate-instructing capacity of Pdx1 as a β-cell selector are inferred from its enriched expression in embryonic and mature β cells. Ectopic Pdx1 alone can induce incomplete reprogramming of liver or pancreatic acinar cells (e.g., Ferber et al. 2000; Heller et al. 2001). A synergistic effect between Pdx1, Neurog3, and MafA was observed when acinar cells were converted into β-like cells (Zhou et al. 2008), which inefficiently ameliorated hyperglycemia caused by loss of endogenous β cells, perhaps because the reprogrammed cells did not assemble into islet-like clusters. Rather than triggering a redirection into endocrine cells, forced Pdx1 expression in Ptf1a-expressing cells caused late stage acinar-to-ductal hyperplasia (Miyatsuka et al. 2006). While these studies suggest that Pdx1 alone is contextually sufficient to induce partial trans-differentiation or trans-determination, little is known about how different competence states affect the response to this single factor.
Here, we report on the previously unknown sufficiency for Pdx1 as a potent regulator of endocrine lineage allocation and maintenance of the mature state. With Pdx1 expression enforced from the Neurog3+ endocrine progenitor state onward, two periods of dominant lineage redirection occurred: (1) during early organogenesis, a minor reproducible reduction in cells directed to the α fate, and (2) a surprising peri/postnatal redirection of Pdx1-expressing α cells, with rapid reprogramming into Ins+ cells that are indistinguishable from normal β cells. The delayed conversion occurred despite α cells having expressed exogenous Pdx1 from their endocrine commitment point onward, suggesting the possibility of a cryptic chromatin-priming effect. In contrast, exogenous Pdx1 in Gcg+ embryonic or adult α cells suppressed Gcg expression but did not induce α/β fate switching. Our findings reveal differential α-to-β plasticity between endocrine progenitors and hormone-secreting cells in response to Pdx1. We speculate on the epigenetic ramifications of these differential lineage-switching findings.