Mechanism of plus-end targeting by the kinesin-8 Kif18A
A Tethering Mechanism Controls the Processivity and Kinetochore-Microtubule Plus-End Enrichment of the Kinesin-8 Kif18A
Jason Stumpff2, 4, Yaqing Du1, 4, Chauca A. English1, Zoltan Maliga3, Michael Wagenbach2, Charles L. Asbury2, Linda Wordeman2, 4, , and Ryoma Ohi1, 4,
1 Department of Cell and Developmental Biology, Vanderbilt University Medical Center, Nashville, TN, 37232
2 Department of Physiology and Biophysics, University of Washington School of Medicine, Seattle, WA, 98195
3 Max Planck Institute of Molecular Cell Biology and Genetics, Dresden, Germany
Received 19 April 2011;
revised 23 June 2011;
accepted 28 July 2011.
Published: September 1, 2011.
Available online 1 September 2011.
Refers to:
Mechanisms Underlying the Dual-Mode Regulation of Microtubule Dynamics by Kip3/Kinesin-8
Molecular Cell, Volume 43, Issue 5, 2 September 2011, Pages 751-763,
Xiaolei Su, Weihong Qiu, Mohan L. Gupta Jr., José B. Pereira-Leal, Samara L. Reck-Peterson, David Pellman
PDF (1556 K) | Supplementary content
Referred to by:
Mechanisms Underlying the Dual-Mode Regulation of Microtubule Dynamics by Kip3/Kinesin-8
Molecular Cell, Volume 43, Issue 5, 2 September 2011, Pages 751-763,
Xiaolei Su, Weihong Qiu, Mohan L. Gupta Jr., José B. Pereira-Leal, Samara L. Reck-Peterson, David Pellman
PDF (1556 K) | Supplementary content
Summary
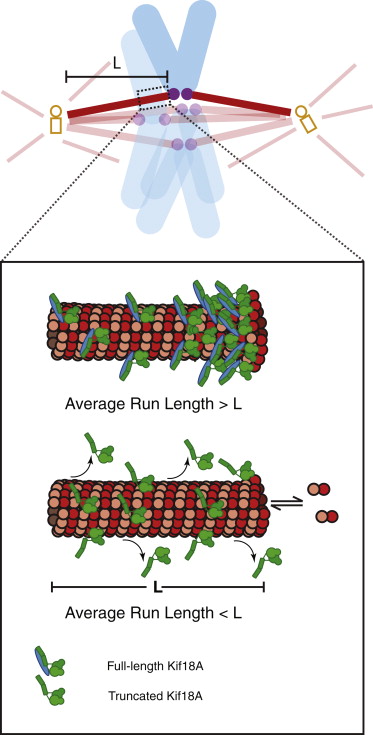
Metaphase chromosome positioning depends on Kif18A, a kinesin-8 that accumulates at and suppresses the dynamics of K-MT plus ends. By engineering Kif18A mutants that suppress MT dynamics but fail to concentrate at K-MT plus ends, we identify a mechanism that allows Kif18A to accumulate at K-MT plus ends to a level required to suppress chromosome movements. Enrichment of Kif18A at K-MT plus ends depends on its C-terminal tail domain, while the ability of Kif18A to suppress MT growth is conferred by the N-terminal motor domain. The Kif18A tail contains a second MT-binding domain that diffuses along the MT lattice, suggesting that it tethers the motor to the MT track. Consistently, the tail enhances Kif18A processivity and is crucial for it to accumulate at K-MT plus ends. The heightened processivity of Kif18A, conferred by its tail domain, thus promotes concentration of Kif18A at K-MT plus ends, where it suppresses their dynamics to control chromosome movements.
Graphical Abstract