By Emily Overway
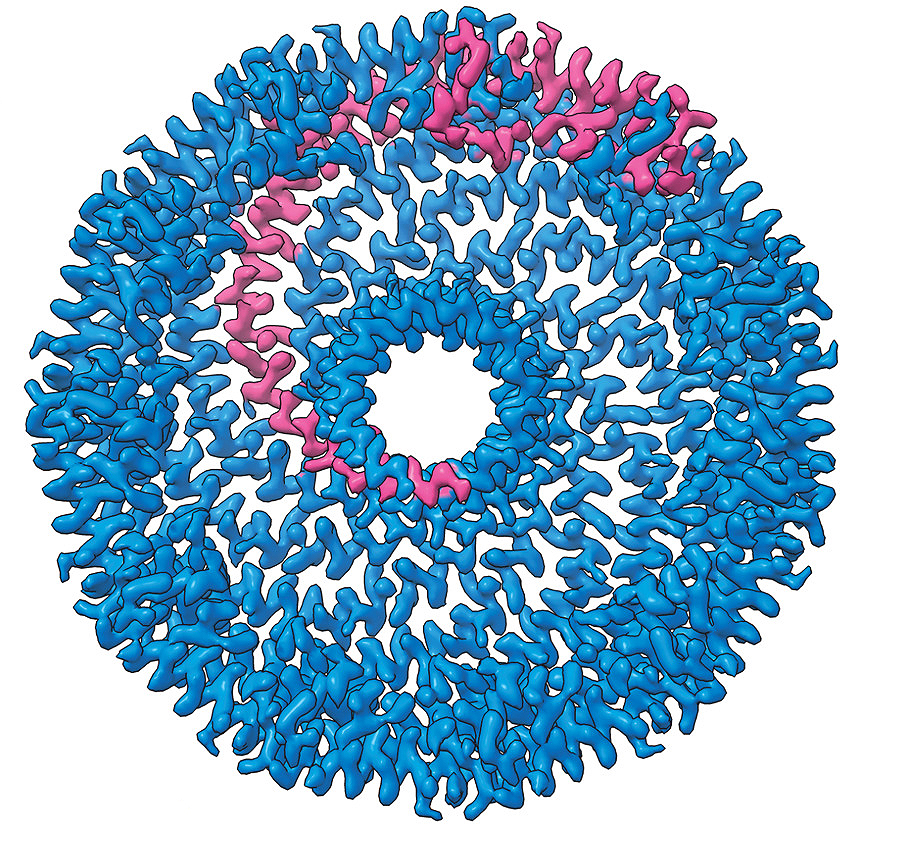
Recent collaborative research, published in Science Advances, used cryo-electron microscopy to determine the structure of caveolin-1 and how many individual caveolin-1 proteins join to form a complex. Caveolin-1 proteins bend the plasma membrane to form indentations known as caveolae, which sense and respond to changes in membrane tension.
The labs of Hassane Mchaourab, the Louise B. McGavock Chair and professor of molecular physiology and biophysics; Jens Meiler, professor of chemistry; and Erkan Karakas, professor of molecular physiology and biophysics, worked with the labs of Melanie Ohi at the University of Michigan and Anne Kenworthy at the University of Virginia to investigate the molecular architecture of the human caveolin-1 complex. Both Ohi and Kenworthy are former Vanderbilt faculty members.
We sat down with Karakas to learn more about this research.
What issue/problem does your research address?
Caveolin proteins form complexes that interact with the plasma membrane to generate essential curvatures in the membrane called caveolae. Caveolae are used to sense and respond to changes in plasma membrane tension. How caveolin proteins generate caveolae and their precise interactions with the plasma membrane are not fully understood. This research determined the first high-resolution structure of the human caveolin-1 complex using cryo-EM, providing unprecedented insight into the molecular basis for caveolin’s membrane-remodeling activity.
What was unique about your approach to the research?
This research utilized that expertise of five labs across three universities. This collaborative effort afforded unique perspectives and techniques that were necessary to accomplish this research.
What were your findings?
The structural insights gained from cryo-EM suggest a new mechanism for how membrane-sculpting proteins like caveolin-1 interact with membranes. Additionally, these findings revealed how different regions of caveolin-1 contribute to its function. The study allowed us to visualize the complex at atomic resolution and identify unique features that were not possible to predict using other methods.
What do you hope will be achieved with the research results in the short and long terms?
We expect this study to be a template for the design of new experiments that will fully characterize this class of proteins; in mammals, there are two other caveolin proteins. In the long term, it may lead to the development of new therapeutic tools allowing us to regulate caveolae function and formation and correct defects caused by dysfunctional caveolin proteins.
What are the benefits of this research?
Caveolin-1 dysfunction in humans has been linked to cardiovascular disease, cancer, lipodystrophy, and kidney disease. By identifying how caveolin-1 interacts with the membrane, this research may spur development of new therapeutic tools against the conditions resulting from defects in caveolin.
Where is this research taking you next?
The next steps in this research are to investigate the molecular assembly of caveolae and the roles of caveolin complexes in caveolae formation. How caveolins are assembled into complexes, how they interact with the membrane, and how they change the shape of the membrane are all questions we are interested in answering with future studies.
Funding
This work was funded by the National Institutes of Health and the Humboldt Professorship of the Alexander von Humboldt Foundation.