ASPIRE Internship: VICTR, Drug Repurposing and Scientific Project Management Internship (Remote)
ASPIRE Internship Opportunity:
VICTR (the Vanderbilt Institute for Clinical and Translational Research)
Drug Repurposing and Scientific Project Management Internship (Remote)
(January – March 2025)
Nashville, TN
Are you a biomedical science graduate student interested in gaining clinical trial research experience? An internship with VICTR’s (the Vanderbilt Institute for Clinical and Translational Research) Drug Repurposing and Scientific Management Team might be what you’re looking for! This internship will provide a basic, working knowledge of clinical and translational research, clinical trial design, execution and management, as well as unique exposure to clinical trials being conducted at Vanderbilt.
About the Team
 VICTR’s Drug Repurposing and Scientific Management Team leads clinical and translational research projects and teams at Vanderbilt.
VICTR’s Drug Repurposing and Scientific Management Team leads clinical and translational research projects and teams at Vanderbilt.
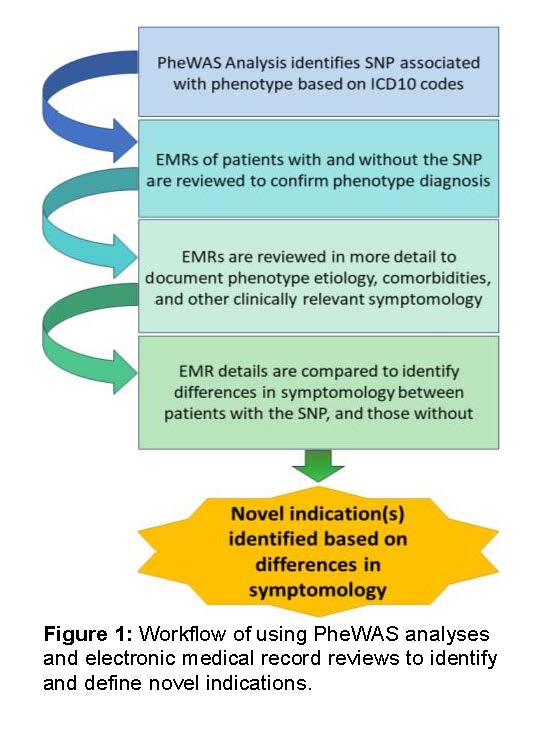
VICTR’s Drug Repurposing program leverages unique Vanderbilt resources, including BioVU and the Synthetic Derivative, for Phenome Wide Association Studies (PheWAS) that identify diseases associated with specific genetic variants. Then, we investigate drugs (with established safety profiles) that mimic the genetic variants in the context of those associated diseases (Figure 1). We work with clinician experts to design and execute the clinical trials needed to test whether the drugs can be repurposed for a new indication.
VICTR’s Scientific Project Management team supports a wide array of projects including single-site pilot studies to large, multi-site clinical trials in multiple therapeutic areas. Our PhD-level project managers help harmonize, design, and execute scientific, operational, and administrative activities to ensure the studies are scientifically robust and successfully completed.
Internship Details and Responsibilities
Introduction to Drug Repurposing and Scientific Project Management
Interns will be introduced to VICTR’s Drug Repurposing program and Scientific Project Management team through a variety of means, including attending one-on-one and group project meetings and reviewing written materials. Through attending these meetings, taking notes, and reviewing key documents (including protocols, manuscripts, SOPs, and other background information), interns will gain foundational knowledge of the conduct of clinical research studies.
Although many members of the Drug Repurposing and Scientific Project Management team completed their PhDs in a basic science field, their current work is vastly different from bench work. Since the terms “clinical research” and “scientific project management” can be broad and nebulous, we believe in setting a solid foundation in clinical research through exposure to the variety of work being completed.
Intern(s) will gain a basic understanding of:
- The overall life cycle of a clinical trial (including a basic knowledge of what happens at each step)
- Key steps needed for start-up and conduct of small, single-site (i.e., “proof-of-concept”) clinical trials
- Key steps needed for start-up and conduct of large, multi-site clinical trials
- What the Institutional Review Board (IRB) is, the role it plays in clinical research, and how it works at VUMC
- Resources available at VUMC to support clinical trials
Responsibilities:
- Reading/reviewing papers and other background materials, SOPs, etc.
- Attending (or reviewing video recording of) 1-2 clinical trial-related meetings per week
- Notetaking for 1-2 clinical trial-related meetings per week
INTERNSHIP DETAILS:
- Internship will run from January-March 2025, with potential to extend pending interest and needs of the organization. Extensions will be re-evaluated every 12 weeks to ensure the internship remains a good fit for both parties.
- Internship is remote, unpaid, part-time (8-10 hours per week, variable).
- A weekly team meeting take place via Zoom on Wednesday 10-11am CST; it would be ideal for the intern to be available for this meeting.
Opportunities for Growth and Advancement toward Ownership of Project Components
If the internship is extended, the intern will have additional opportunities to gain a more thorough understanding of key aspects of clinical trials, such as:
- Regulatory reporting
- Operations management (IRB amendments, etc.)
- Collection and management of study data
- Intellectual Property and External/Industry Partnerships
Responsibilities
- Supporting individual trial tasks and projects (i.e., drafting content for IRB submissions, drafting slides for Operations/team meetings, building study database instruments in REDcap, etc.)
Due to the nature of the work conducted by the Drug Repurposing and Scientific Project Management team, new projects may develop sporadically. These new projects can present opportunities for the intern(s) to complete novel tasks at the discretion of the team such as:
- De-identified medical chart reviews using the Synthetic Derivative
- Literature reviews around project-specific topics
- Drafting content for invention disclosures
Key Skills Gained from this Internship
- Clinical trial design, development, and management
- Knowledge of clinical trial recruitment and retention design and development
- Basic understanding of key regulatory aspects of clinical trials
- Basic understanding of clinical trial operations
- Exposure to effective meeting leadership and management techniques
- Effective written communication skills, including distillation of complex discussions into valuable summaries and action items
- Knowledge of scientific project management approaches in a matrixed environment
Ideal Candidate
The ideal candidate is a graduate student or postdoctoral fellow interested in learning more about project management, clinical research, and career opportunities within the clinical research space.
Applications due by noon, Monday, October 28, 2024
To apply:
- Fill out the following ASPIRE Internship Application found here: https://redcap.link/ASPIREInternship_Application
- Submit a resume and cover letter at the RedCap link above. Please submit documents as pdfs with your last name_VICTR_Resume_Sp 2025 or last name_VICTR_coverletter_Sp 2025
- Set up a meeting with Angela Zito at (angela.zito@vanderbilt.edu) prior to the application deadline.
Your information will then be passed along to the organization who will contact you directly to schedule an interview.
For further questions, please contact Angela Zito (angela.zito@vanderbilt.edu) or Ashley Brady (ashley.brady@vanderbilt.edu).
——————-
Please review ASPIRE Internship FAQs: Found here
For further questions, please contact Ashley Brady (ashley.brady@vanderbilt.edu).
Eligibility requirements
- Applicants must be a current Vanderbilt University PhD student or a current postdoctoral fellow.
- All PhD students must have completed all coursework for their degree prior to the start of the internship.
- Biomedical PhD students must also have passed their qualifying exams before the application deadline.
- Students and postdocs may not participate in more than one internship at a time. More than one application may be submitted at a single time, but only one position can be accepted.
- If you are not in a department supported by the BRET office, we will also require that you complete a form guaranteeing PI approval to participate in this program. Please contact Ashley Brady at ashley.brady@vanderbilt.edu for more information.
- If you are an international student or postdoctoral fellow, your visa status may affect your work eligibility. Please contact Ashley Brady (ashley.brady@vanderbilt.edu) to discuss this further.
- Must be authorized to work in the US